
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Seven-carbon ring compound |
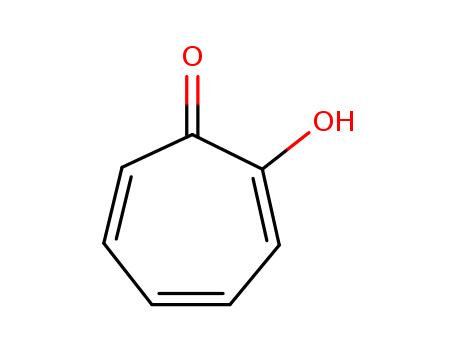
Tropolone, also known as tohenone and 2-hydroxygenone, is a kind of seven-carbon ring compound, which is weakly acidic and has the properties of aromatic compounds, double bond and weak ketone. There are more than ten kinds of compounds known in nature containing seven-carbon rings: Hinokitiol, as a red iron complex, is found in cypress wood in Taiwan. Alpha- and gamma-thujapricin (β-body is the same as hinokitiol) are found in cypress and coniferous plants. It acts as an antibacterial agent to the wood for anticorrosion α- body and β-body also exist in the cypress essential oil. Stipitatic acid (6- hydroxyaryl heptanone-4-carboxylic acid) is the metabolite of penicillium, and has an antibacterial effect. Purpurogallin, as glycoside, is found in the galls of mistletoe plants and can be used as a phenol oxidase test. As nootkatin of the nootka heartwood and subalkaloids of colchicine and the like of colchicum used as inhibitor. According to the properties of the compounds above, the scientists speculate that tocophenone plays an important role in the metabolic process of living components. It wss Japanese scientists Tetsuo Nozoe who first paid attention to tropolone in 1936 when he was researching hinokitiol, while the work in Europe and the United States started from the structure of stipitatic acid and colchicine, going further after 1950. |
|
Toxicity |
The acute oral toxicity LD50 in rats was 459 mg / kg (female) , 223 mg / kg (male) and (662 mg / kg, rat). The acute dermal toxicity LD(50) in rabbit was no less than 2000mg/kg. There was mild irritation to the eye of rabbits. LC50 in Japanese carp is 0.5mg / L (48h) . An improved test and hamster ovary test, done by Ames, showed no mutations had been caused. Japanese carp LC50 is 0.5mg / L (48h) |
|
Flammability and Explosibility |
Notclassified |
|
Synthesis |
To a mixture of sodium hydroxide (33 g) with glacial acetic acid (270 ml) was added dropwise via funnel 7,7-dichlorobicyclo[3,2,0]hepta-2-ene-6-ketone (33.5 g) under nitrogen, and the mixture was heated to reflux for 8 hours. After PH was adjusted to 1 with hydrochloric acid (50 ml), the mixture was filtered and extracted with benzene (3 × 90ml). The combined extracts were concentrated in vacuo to give brownish black oil.Fraction of reduced pressure distillation at 100 °C/67 Pa was collected to give gross product as a pale yellow solid. Recrystallization from mixture of dichloromethane and pentane (1/4, V/V) gave analytically pure product Tropolone(17.8 g,77%) as a white needle crystal, m.p. 50~51 °C. |
|
Purification Methods |
Crystallise tropolone from hexane or pet ether and sublime it at 40o/4mm. Also distil it at high vacuum. [Beilstein 8 IV 159.] |
InChI:InChI=1/C7H6O2/c8-6-4-2-1-3-5-7(6)9/h1-5H,(H,8,9)
Four types of tris-chelate ruthenium com...
Xanthine oxidase (XO) is an enzyme that ...
A series of self-immolative boronic este...
Set forth herein, inter alia, are compos...
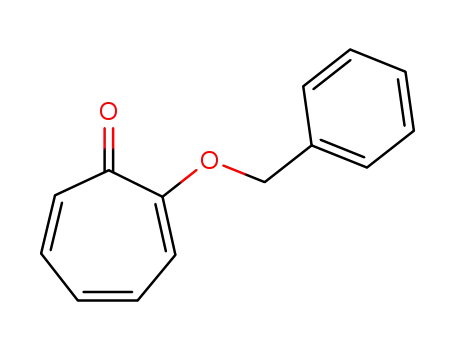
2-benzyloxycyclohepta-2,4,6-trienone

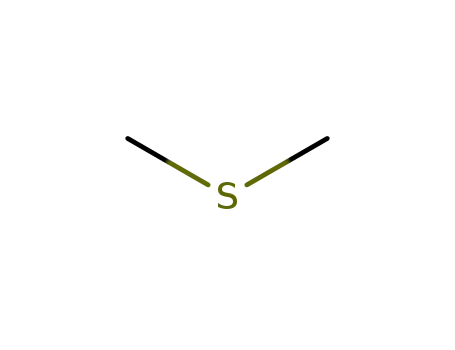
dimethylsulfide

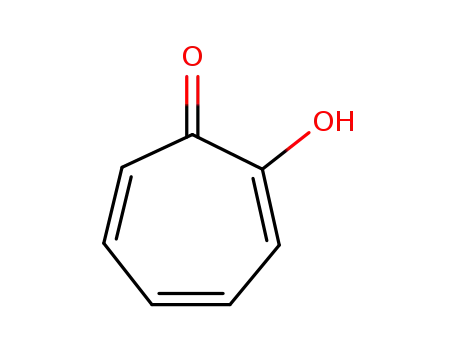
2-hydroxy-2,4,6-cycloheptatrien-1-one

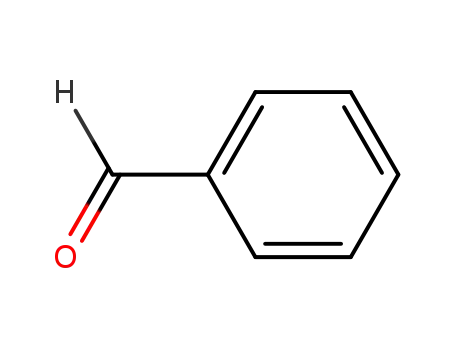
benzaldehyde
| Conditions | Yield |
|---|---|
|
With
dimethyl sulfoxide;
at 180 ℃;
for 2h;
other troponyl ethers;
|
82% |
![(+/-)-7,7-dichlorobicyclo[3.2.0]hept-2-en-6-one](/upload/2025/4/50821769-603c-4dc1-b594-5574514e1d13.png)
(+/-)-7,7-dichlorobicyclo[3.2.0]hept-2-en-6-one


2-hydroxy-2,4,6-cycloheptatrien-1-one
| Conditions | Yield |
|---|---|
|
With
acetic acid; sodium hydroxide;
|
95% |
|
With
sodium hydroxide; acetic acid;
at 130 ℃;
for 7h;
|
87% |
|
With
hydrogenchloride; sodium hydroxide; acetic acid;
for 8h;
pH=1;
Heating;
|
77% |
|
With
sodium hydroxide; acetic acid;
for 8h;
Heating;
|
77% |
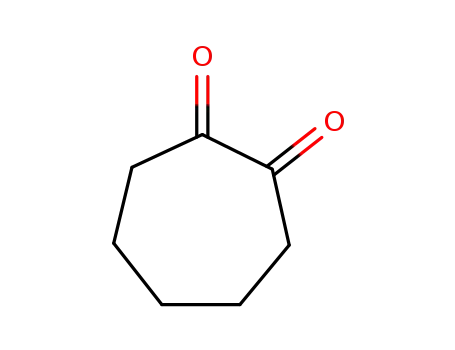
cycloheptane-1,2-dione
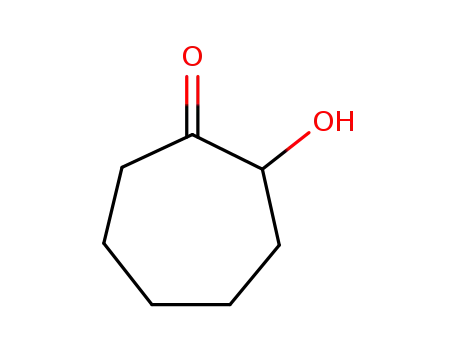
2-hydroxycyloheptanone
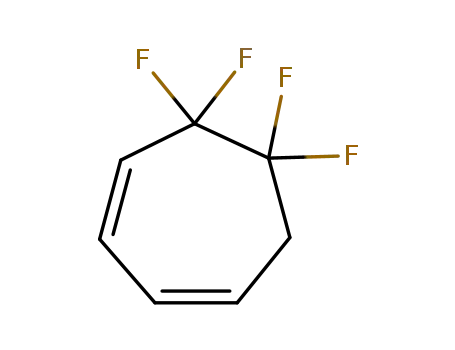
5,5,6,6-tetrafluoro-cyclohepta-1,3-diene
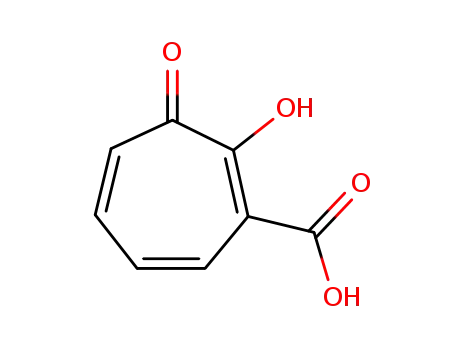
3-carboxytropolone
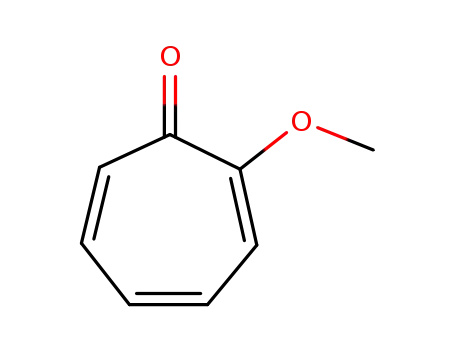
2-methoxytropone
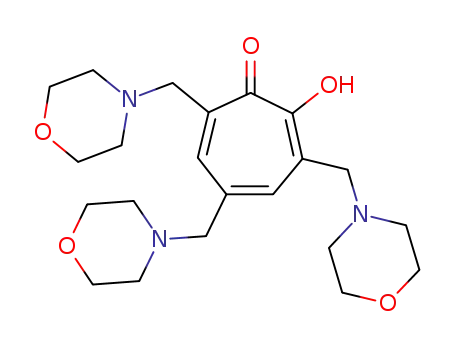
3,5,7-tris(morpholinomethyl)tropolone
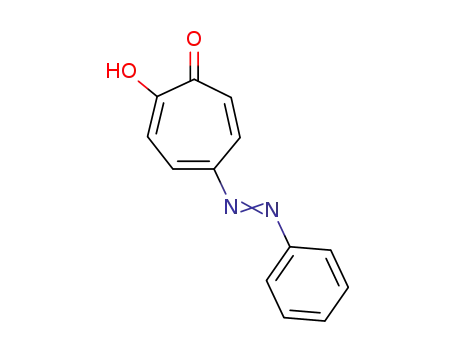
5-phenylazo-tropolone
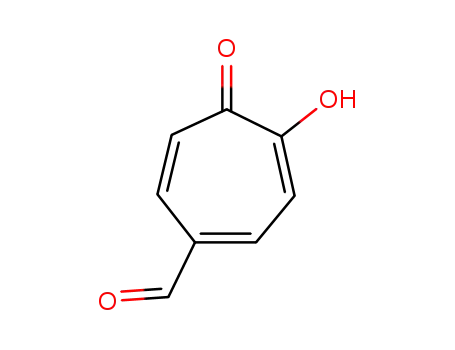
5-formyltroponolate
