
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Purification Methods |
Purify TEMPO by sublimation (33o, water aspirator) [Hay & Fincke J Am Chem Soc 109 8012 1987, Keana Chem Rev 78 37 1978]. |
|
General Description |
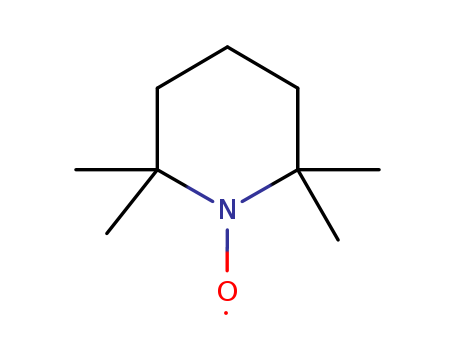
2,2,6,6-Tetramethylpiperidinooxy (TEMPO) is a stable nitroxide radical widely used in catalysis, dynamic nuclear polarization (DNP) for NMR, and polymer chemistry. It exhibits high reactivity in alcohol oxidation and serves as a key component in biradical systems for enhancing DNP efficiency. TEMPO derivatives can be modified for recyclability in catalytic processes or incorporated into stimuli-responsive polymers, demonstrating versatility in both synthetic and materials applications. |
InChI:InChI=1/C9H18NO/c1-8(2)6-5-7-9(3,4)10(8)11/h5-7H2,1-4H3
Bubbling O2 into a THF solution of CoII(...
In contrast to 2,2,6,6-tetramethylpiperi...
A new structurally characterized ferrous...
The effect of viscosity on the radical t...
The heteroleptic (formazanato)nickel bro...
The enzymes manganese lipoxygenase (MnLO...
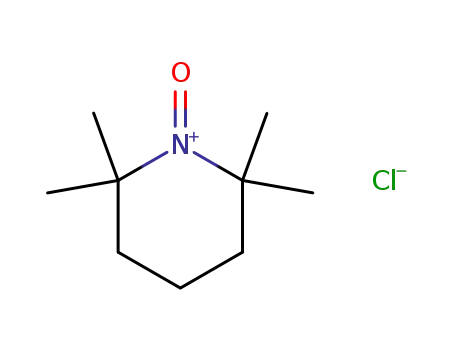
2,2,6,6-tetramethylpiperidin-1-oxoammonium chloride

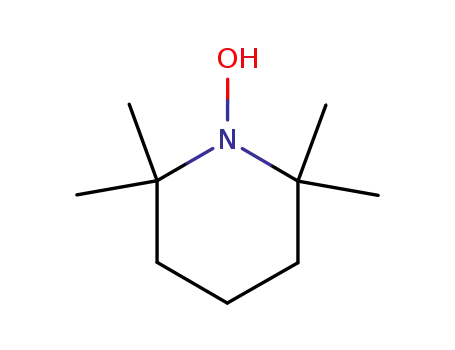
2,2,6,6-tetramethylpiperidin-1-ol

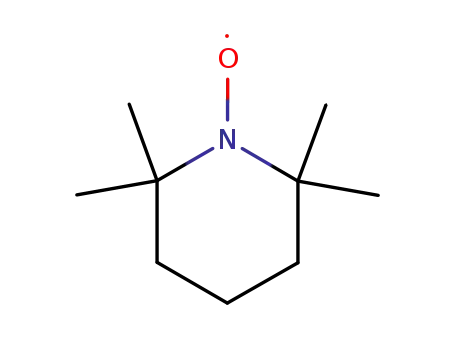
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

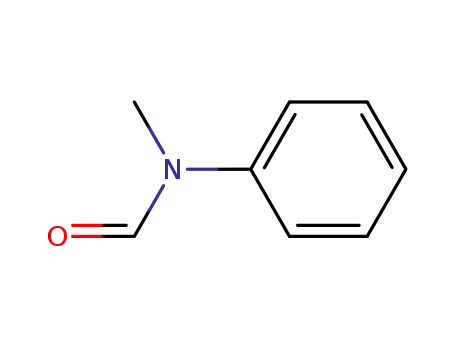
N-methyl-N-phenylformamide

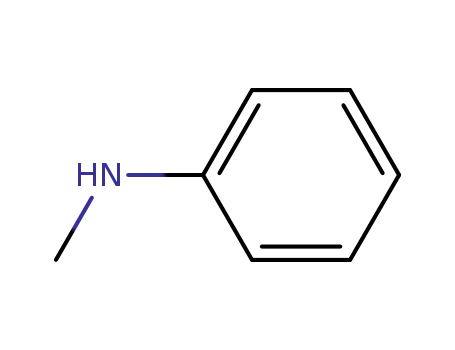
N-methylaniline
| Conditions | Yield |
|---|---|
|
With N,N-dimethyl-aniline; In dichloromethane; at -78 ℃; for 0.5h; Further byproducts given;
|

2,2,6,6-tetramethylpiperidin-1-oxoammonium chloride

N,N-dimethyl-aniline

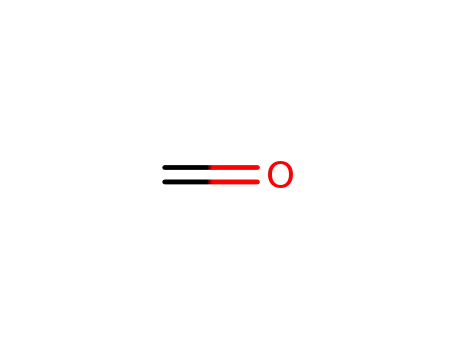
formaldehyd


2,2,6,6-tetramethylpiperidin-1-ol


2,2,6,6-Tetramethyl-1-piperidinyloxy free radical


N-methyl-N-phenylformamide

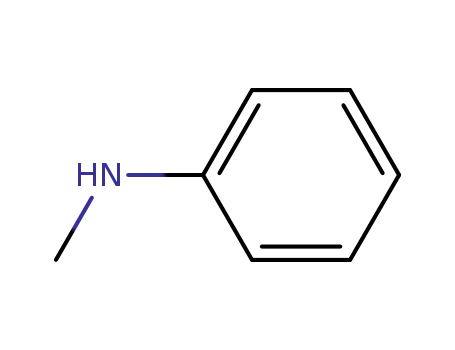
N-methylaniline
| Conditions | Yield |
|---|---|
|
In dichloromethane; at 0 ℃; for 0.5h; Product distribution; Mechanism; var. ratio of oxoimonium ion/substrate, reactions at -80 deg C, var. N,N-dialkylanilines;
|
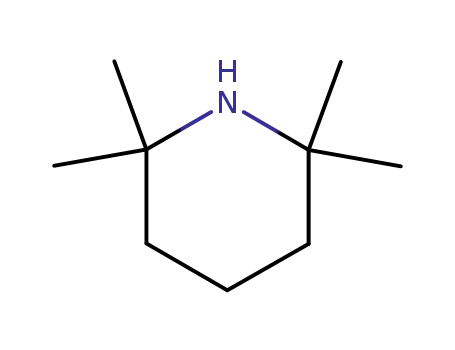
2,2,6,6-tetramethyl-piperidine
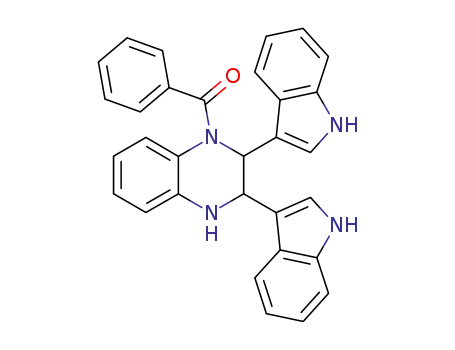
1-benzoyl-2,3-di-indol-3-yl-1,2,3,4-tetrahydro-quinoxaline

2,2,6,6-tetramethylpiperidin-1-ol

2,2,6,6-tetramethylpiperidin-1-oxoammonium chloride
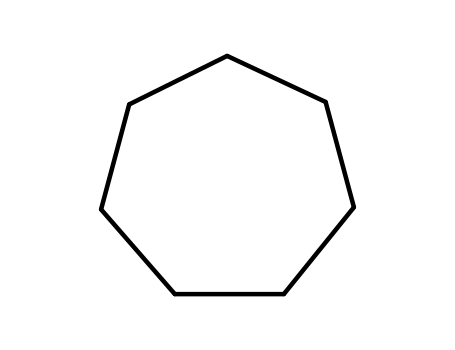
cycloheptane
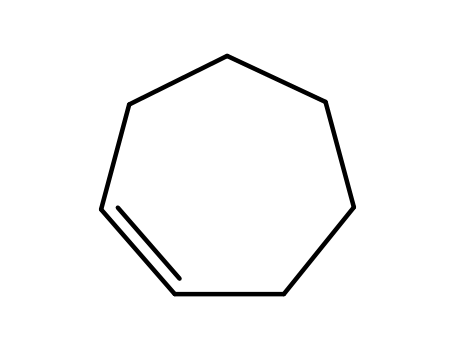
Cycloheptene
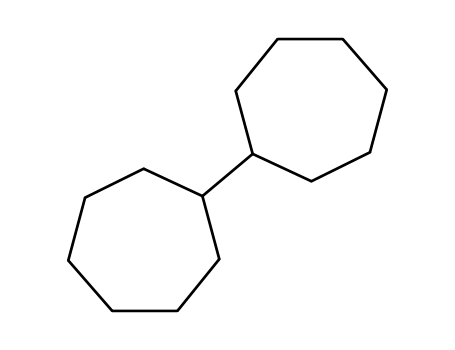
bicycloheptane
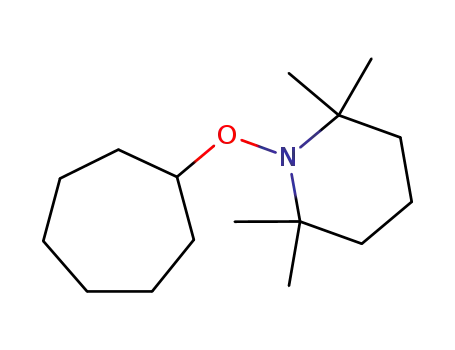
1-(cycloheptyloxy)-2,2,6,6-tetramethylpiperidine
