
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Flammability and Explosibility |
Nonflammable |
|
Synthesis |
Alkyl bromide and sodium azide were used as raw materials to prepare organic azides, and a series of 1,2,4-Triazole small molecules were prepared by click chemistry. The structures of the six products were characterized by 1H NMR, 13C NMR and IR. the products were expected targets. Furthermore, the effect of different substituent structure on the reaction was discussed. Especially the introduction of drug molecule ethisterone, which is expected to provide a certain experimental basis for the application of triazole and its derivatives in biomedicine and other fields.As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3. |
|
Purification Methods |
Crystallise 1,2,4-triazole from EtOH, H2O, EtOAc (m 120.5-121o), or EtOH/*C6H6. The hydrochloride has m 170o, and the picrate has m 163-164o (from H2O or CHCl3). [Barszcz et al. J Chem Soc, Dalton Trans 2025 1986]. [Beilstein 26 H 13, 26 II 6, 26 III/IV 35.] |
|
Chemical Composition and Structure |
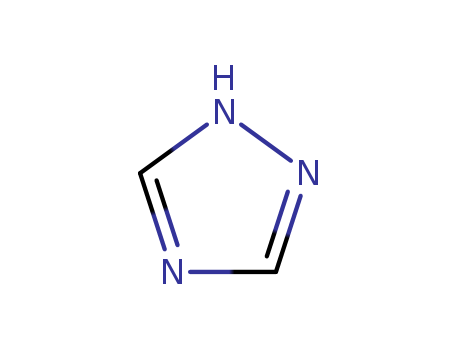
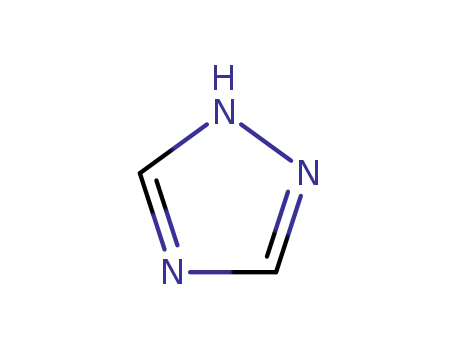
1,2,4-Triazole is an organic compound characterized by its five-membered ring structure containing three nitrogen atoms at positions 1, 2, and 4. It is an important pharmacophore in various bioactive compounds due to its stability and ability to interact with receptors as hydrogen bond acceptors and donors. |
|
Mechanism of Action |
The mechanism of action varies depending on the specific derivative and its intended application. However, 1,2,4-Triazole compounds often exert their biological activities by interacting with specific molecular targets, such as enzymes or receptors, thereby modulating their function. |
|
Production Methods |
The synthesis of 1,2,4-Triazole and its derivatives involves various methods, including the formamide method and cyclization reactions. Transition metal-catalyzed methods are also employed for efficient and direct synthesis. |
|
Analysis Method |
Analytical techniques such as mass spectrometry are commonly used to characterize and identify 1,2,4-Triazole derivatives, including determination of their molecular ion peaks and fragmentation patterns. |
|
General Description |
1,2,4-Triazole is a versatile heterocyclic compound with significant pharmacological and chemical applications. It serves as a key scaffold in the synthesis of bioactive derivatives, including Schiff bases, azetidinones, and metallophthalocyanines, demonstrating antimicrobial, antitubercular, anticonvulsant, and analgesic properties. Its structural adaptability allows for efficient green and microwave-assisted syntheses, yielding high-purity compounds with potential drug-like characteristics. Additionally, 1,2,4-triazole derivatives exhibit thermal stability and are utilized in optical sensing and magnetochemical applications. The compound's reactivity enables diverse functionalization, making it valuable in medicinal chemistry and material science. |
|
Definition |
1,2,4-triazole is a chemical substance with molecular formula C2H3N3, molecular weight 69.07 and CAS No. 288-88-0. Colorless acicular crystal, soluble in water and ethanol. 1H-1,2,4-triazole is a 1,2,4-triazole. It is a tautomer of a 3H-1,2,4-triazole and a 4H-1,2,4-triazole. Triazole is an aromatic five membered heterocyclic compound. Its unique structure is conducive to the formation of a variety of non covalent bonds and is easy to promote the binding of proteins, enzymes and receptors, thus showing a wide range of biological pharmacological activities, such as anticancer, antispasmodic, antibacterial, anti HIV. In particular, 1,2,3-triazole has strong thermal stability and acid-base stability, and is not sensitive to redox, hydrolysis and enzymatic degradation. It is often used as a pharmacodynamic group in drug synthesis. |
InChI:InChI=1/C2H3N3/c1-3-2-5-4-1/h1-2H,(H,3,4,5)
Kinetic data are reported for the sponta...
Binding of sodium dodecyl sulfate (SDS) ...
Kinetic medium effects are presented for...
Rates and thermodynamic activation param...
At temperatures above and below the temp...
4-(and 1-) Amino-1,2,4-triazoles have be...
Rates and thermodynamics activation para...
Kinetic data are reported for hydrolysis...
The water-catalyzed hydrolysis of 1-benz...
The rate of the water-catalyzed hydrolys...
-
Pseudo-first-order rate constants and th...
Highly electrophilic 6-nitro-4-azabenzof...
The invention belongs to the technical f...
N-Acyl imidazoles and catalytic isothiou...
The invention discloses a synthesizing p...
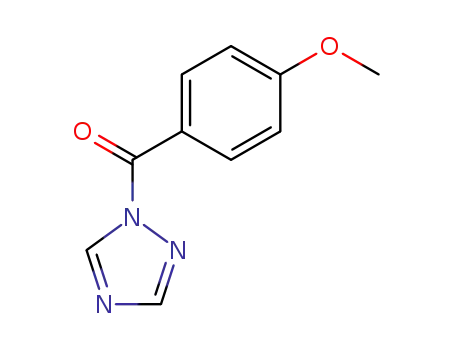
1-(p-anisoyl)-1,2.4-triazole

1,2,4-Triazole

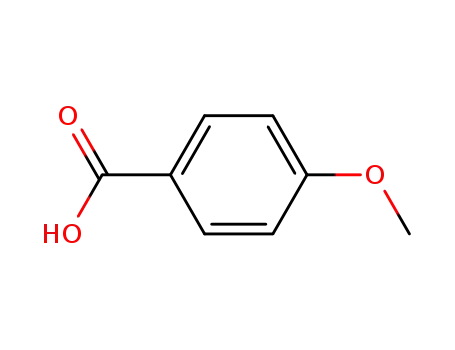
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With
water;
In
tert-butyl alcohol;
at 25 ℃;
Rate constant;
kinetic solvent effect; hydrolysis;
|
|
|
With
hydrogenchloride; water; cetyltrimethylammonim bromide;
at 25 ℃;
Further Variations:;
Reagents;
Kinetics;
|
|
|
With
water; sodium dodecyl-sulfate;
In
acetonitrile;
at 25 ℃;
pH=5.3;
Concentration;
Reagent/catalyst;
Time;
pH-value;
Kinetics;
Micellar solution;
|
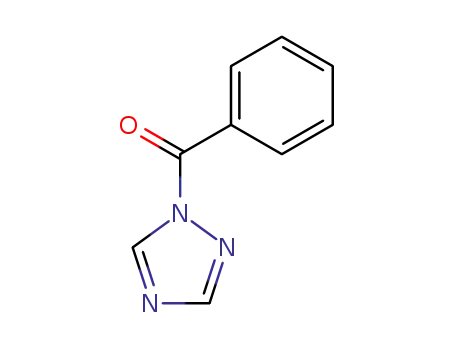
1-benzoyl-1,2,4-triazole


1,2,4-Triazole

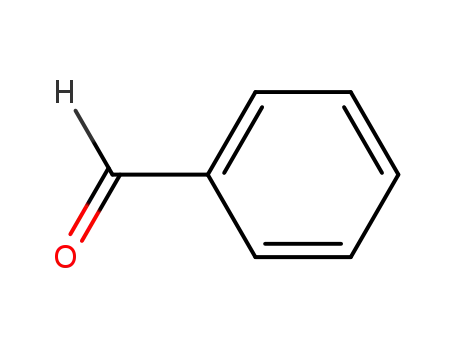
benzaldehyde
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; tetraethylammonium bromide; water;
In
acetonitrile;
at 25 ℃;
pH 4; kinetic medium effects of cationic cosolutes on the neutral hydrolysis; further ammonium bromides;
|
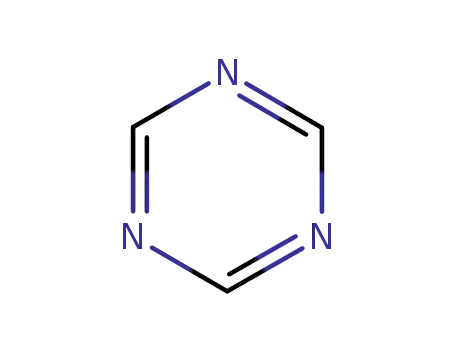
1,3,5-Triazine
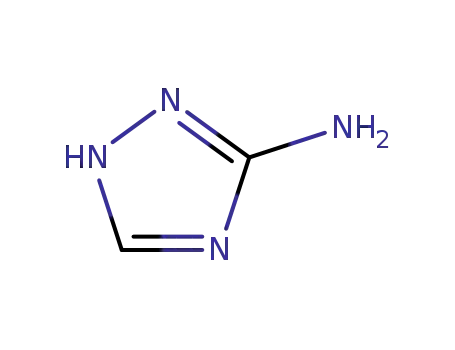
3(5)-amino-1,2,4-triazole
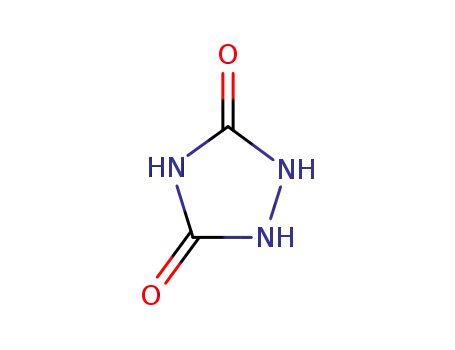
urazole
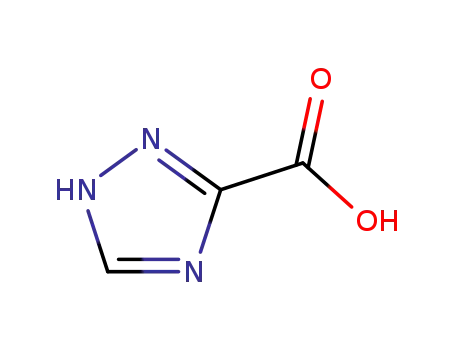
1,2,4-triazole-3-carboxylic acid
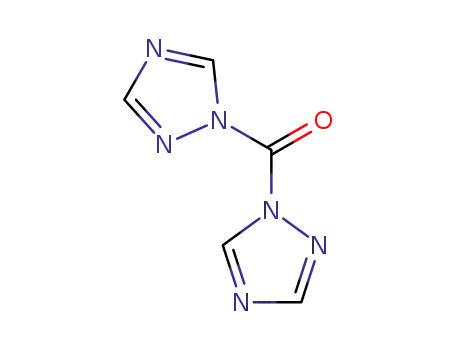
1,1'-Carbonyl-di-(1,2,4-triazole)
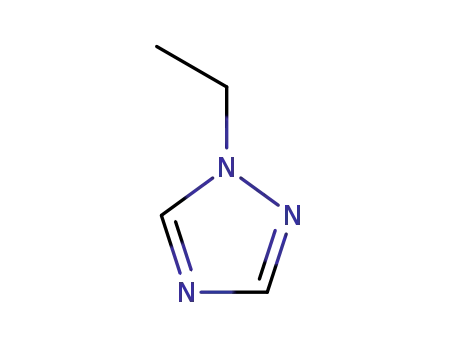
1-ethyl-1H-1,2,4-triazole
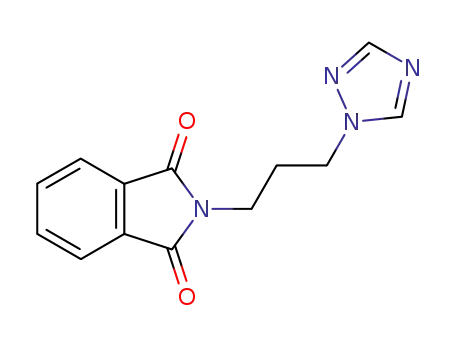
N-(3-[1,2,4]triazol-1-yl-propyl)-phthalimide
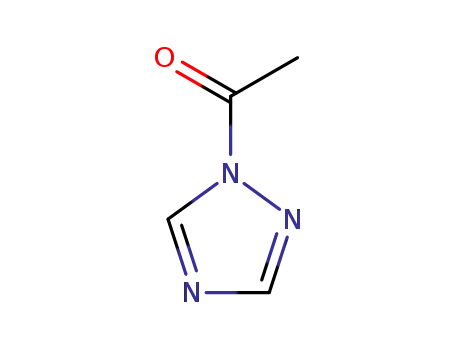
1-acetyl-1H-[1,2,4]triazole
