
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
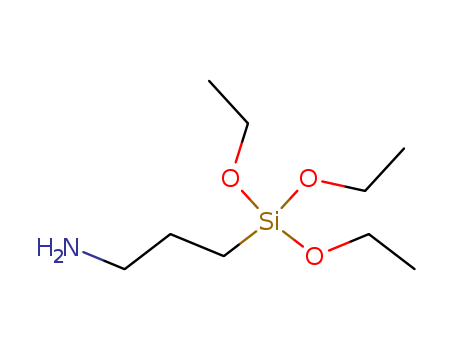
Silane coupling agent KH-550 |
3-Aminopropyltriethoxysilane is the earliest widely used coupling agent, so far has been more than 40 years of history. One end of the structure is provided with an active group, such as amino and vinyl, which can react with synthetic resin molecules such as epoxy, phenolic, polyester. The other end is alkoxy (such as methoxy, ethoxy etc.) or chlorine atoms which connected with silicon, and these groups. These groups can react with the hydroxyl groups on the surface of glass, minerals, inorganic fillers and generate reactive silicon alcohol in the presence of water in the aqueous solution or air. Therefore, silane coupling agent is often used in silicate-filled epoxy, phenolic, polyester resin and so on. In addition, it can also be used in the production of glass fiber reinforced plastic, in order to improve its mechanical strength and resistance to wet environment. The organic group of the silane coupling agent has selectivity to the reaction of the synthetic resin. Generally, these organic groups are insufficiently reactive with synthetic resins such as polyethylene, polypropylene, polystyrene and so on, so that the coupling effect is poor. In recent years, new types of silane coupling agents have been developed which have a good coupling effect on polyolefins, but it is not widely used in cost and other properties. Silane coupling agent, also known as silane treatment agent, primer. The general formula is Y (CH2) nSiX3, it is an organic silicon monomer with two or more different reactive groups in the molecule, which can be chemically bonded (coupled) with organic and inorganic materials, and increase the bonding property of the two materials. In the general formula, N is an integer of 0 to 3; X is a hydrolyzable group, such as chlorine, methoxy, ethoxy, acetoxy and the like, and is easily hydrolyzed to form a silanol which can be combined with an inorganic substance; Y is an organic functional group, such as a vinyl group, an amino group, an epoxy group, a methacryloyloxy group, a mercapto group and the like, which can react with organic compounds and combine. The performance of typical silane coupling agent is as follows: For glass fiber, inorganic filler surface treatment. Used as a sealant, adhesive and paint thickener. It also can be used to the immobilized enzyme attached to the surface of glass substrate, to sand control in oil well drilling, to prevent sand from drilling, to brick surface with hydrophobic, to make the fluorescent lamp coating have high surface resistance, and to improve the moisture absorption properties of organic matter on the surface of glass in the medium of liquid chromatography. It is catalyst in the platinum by the chloroform and alkene with the active group? and then obtained by alcoholysis. The molecular structure and application information of silane coupling agent 3-aminopropyltriethoxysilane are edited and edited by chemcialbook andy. |
|
Flammability and Explosibility |
Notclassified |
|
Biochem/physiol Actions |
(3-Aminopropyl)triethoxysilane forms aminopropyl derivative of glass, an adsorbent for affinity chromatography. It is used to prepare positively charged slides suitable for use with various immunohistochemical and in situ hybridization procedures. |
|
Safety Profile |
Poison by intraperitoneal route.Moderately toxic by ingestion and skin contact. A skin andeye irritant. When heated to decomposition it emits toxicfumes of NOx. |
|
General Description |
(3-Aminopropyl)tiethoxysilane (APTES) is used as an amino-silane which is mainly used as a dispersant. APTES attaches an amino group to the functional silane for bio-conjugation. |
InChI:InChI=1/C9H23NO3Si/c1-4-11-14(12-5-2,13-6-3)9-7-8-10/h4-10H2,1-3H3
The subject of the present application i...
Switchable electrolytes, whose propertie...
The subject of the present disclosure is...
It is an object of the present disclosur...
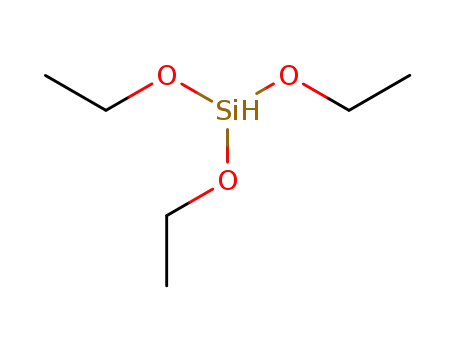
Triethoxysilane

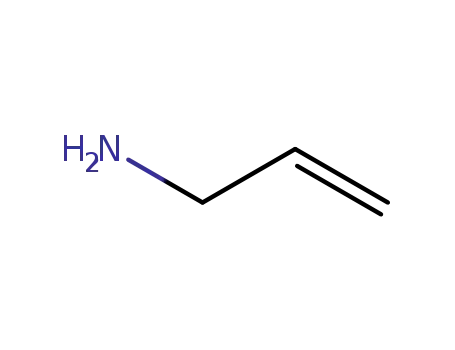
1-amino-2-propene

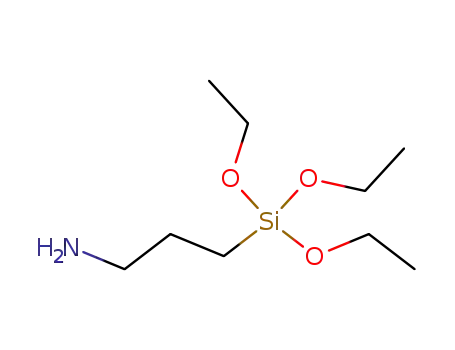
3-aminopropyltriethoxysilane

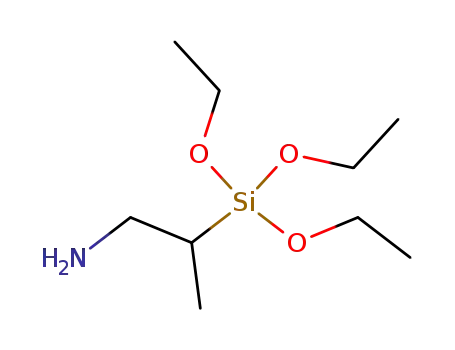
(2-amino-1-methylethyl)triethoxysilane
| Conditions | Yield |
|---|---|
|
With Allyl glycidyl ether; dihydrogen hexachloroplatinate; isopropyl alcohol; for 13h; Product distribution; Mechanism; influence unsaturated Si-comp. on rate of hydrosilylation; effect of other additives;
|
74.8 % Chromat. |
|
With (dicyclopentadiene)platinum(II) dichloride; at 85 ℃; for 20h; Overall yield = 84 %Spectr.; Inert atmosphere; Schlenk technique;
|
|
|
With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex; at 80 ℃; for 20h; Overall yield = 94.2 %Spectr.; regioselective reaction; Schlenk technique; Inert atmosphere;
|

Triethoxysilane


1-amino-2-propene

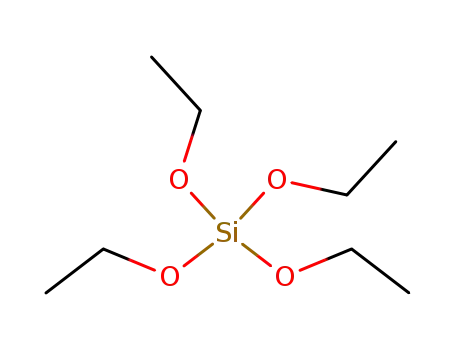
tetraethoxy orthosilicate


3-aminopropyltriethoxysilane
| Conditions | Yield |
|---|---|
|
With dihydrogen hexachloroplatinate; 7,8-dicarba-nido-undecaborate(1-); for 10h; Product distribution; Heating; other reaction time, various catalyst composition, other catalysts;
|
69.9% 11.1% |
|
With dihydrogen hexachloroplatinate; 7,8-dicarba-nido-undecarborate(1-); for 10h;
|
11.1% 69.9% |
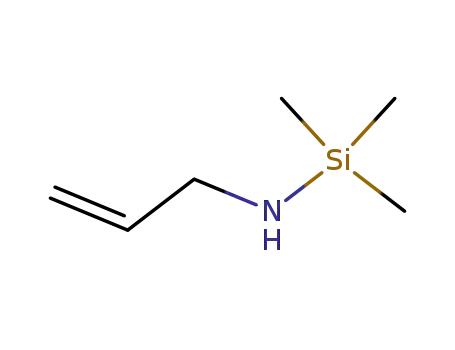
allylamino-trimethyl-silane

Triethoxysilane
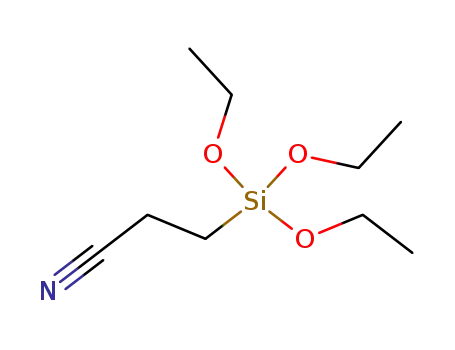
2-cyanoethyltriethoxysilane
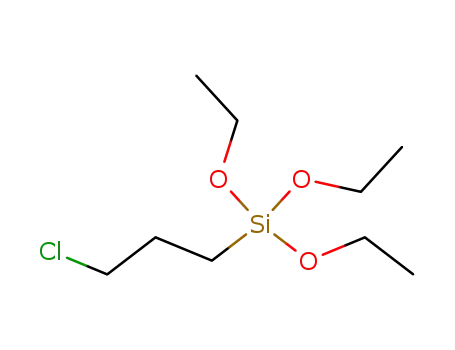
(3-chloropropyl)triethoxysilane
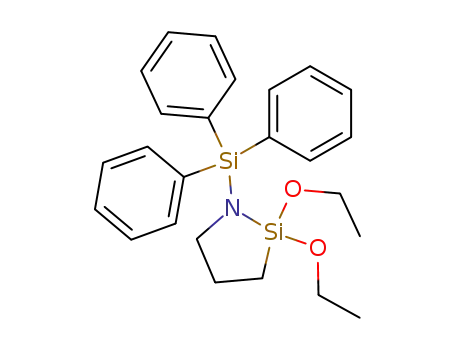
2,2-diethoxy-1-triphenylsilanyl-[1,2]azasilolidine
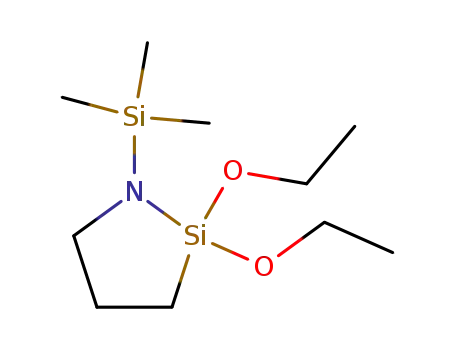
2,2-diethoxy-1-trimethylsilyl-1-aza-2-silacyclopentane
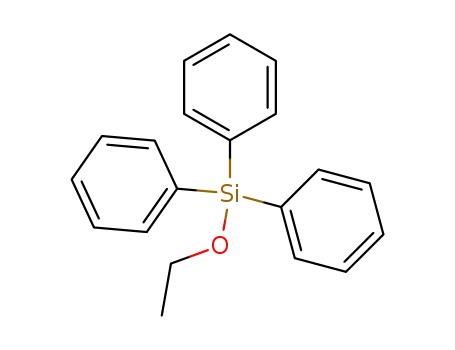
ethoxytriphenylsilane
N, N'-bis<3-(triethoxysilyl)propyl>thiourea
