
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Production |
It can be derived from the esterification of glycerol and acetic acid. After preheating glycerol to 50-60 ° C, add acetic acid, benzene and sulfuric acid. Heat and stir for refluxing dehydration, and recycle the benzene. Then add acetic anhydride for heating of 4h. After cooling, the mixture was neutralized with 5% sodium carbonate to pH 7, and the crude layer was dried and the crude oil was dried with calcium chloride. Distill under reduced pressure, collect the 128-131 ° C (0.93 kPa) fraction, namely glycerol triacetate. |
|
Content analysis |
Accurately weigh about 1g of the sample, put it into a suitable pressure bottle, add 25 mL of 1mol / L. potassium hydroxide solution and 15 mL of isopropyl alcohol, add stopper, wrap with cloth and put it in a canvas bag. Put it into the water bath of 98 ℃ ± 2 ℃ for 1h, and the water level in the water bath should be slightly higher than the bottle level. Take the bottle out from the bag, cool it to room temperature in the air, unfold the cloth and stopper to release the residual pressure in the bottle, and then remove the cloth. Add 6 to 8 drops of phenolphthalein test solution (TS-167), apply 0.5mol / L sulfuric acid for titration of excess alkali until the pink could just disappeared. At the same time, perform a blank test. Each mL of 0.5mol / L sulfuric acid is equivalent to 36.37 mg of glyceryl triacetate (C9H14O6). |
|
Toxicity |
ADI is not subject to special provisions (FAO / WHO, 2001). GR.AS (FDA, § 182.1901, 2000). LD50 3000mg / kg (rat, oral). |
|
Production Methods |
Triacetin is prepared by the esterification of glycerin with acetic anhydride. |
|
Preparation |
By direct reaction of glycerol with acetic acid in the presence of Twitchell’s reagent, or in benzene solution of glycerol and boiling acetic acid in the presence of a cationic resin (Zeo-Karb H) pretreated with dilute H2SO4. |
|
Manufacturing Process |
200 grams of allyl acetate, 450 grams of glacial acetic acid and 3.71 grams of cobaltous bromide were charged to the reactor and the mixture was heated to 100°C. Pure oxygen was then introduced into the reactor below the surface of the liquid reaction mixture at the rate of 0.5 standard cubic feet per hour. Initially, all of the oxygen was consumed, but after a period of time oxygen introduced into the mixture passed through unchanged. During the course of the reaction, a small quantity of gaseous hydrogen bromide (a total of 1.9 grams) was introduced into the reaction zone, along with the oxygen. The reaction was allowed to continue for 6 hours following which the reaction mixture was distilled. Essentially complete conversion of the allyl acetate took place. A yield of 116 grams of glycerol triacetate was obtained, this being accomplished by distilling the glycerol triacetate overhead from the reaction mixture, at an absolute pressure of approximately 13 mm of mercury. |
|
Therapeutic Function |
Topical antifungal |
|
Pharmaceutical Applications |
Triacetin is mainly used as a hydrophilic plasticizer in both aqueous and solvent-based polymeric coating of capsules, tablets, beads, and granules; typical concentrations used are 10–35% w/w. Triacetin is used in cosmetics, perfumery, and foods as a solvent and as a fixative in the formulation of perfumes and flavors. |
|
Contact allergens |
Triacetin is a component of cigarette filters, which induced a contact dermatitis in a worker at a cigarette manufactory. |
|
Safety Profile |
Poison by ingestion. Moderately toxic by intraperitoneal, subcutaneous, and intravenous routes. An eye irritant. Combustible when exposed to heat, flame, or powerful oxidizers. To fight fire, use alcohol foam, water, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Safety |
Triacetin is used in oral pharmaceutical formulations and is generally regarded as a relatively nontoxic and nonirritant material at the levels employed as an excipient. LD50 (dog, IV): 1.5 g/kg LD50 (mouse, IP): 1.4 g/kg LD50 (mouse, IV): 1.6 g/kg LD50 (mouse, oral): 1.1 g/kg LD50 (mouse, SC): 2.3 g/kg LD50 (rabbit, IV): 0.75 g/kg LD50 (rat, IP): 2.1 g/kg LD50 (rat, oral): 3 g/kg LD50 (rat, SC): 2.8 g/kg |
|
storage |
Triacetin is stable and should be stored in a well-closed, nonmetallic container, in a cool, dry place. |
|
Incompatibilities |
Triacetin is incompatible with metals and may react with oxidizing agents. Triacetin may destroy rayon fabric. |
|
Regulatory Status |
GRAS listed. Accepted in Europe as a food additive in certain applications. Included in the FDA Inactive Ingredients Database (oral capsules and tablets and gels). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Chemical properties |
Colorless, odorless oily liquid. It is miscible with ethanol, ether, benzene, chloroform and other organic solvents, soluble in acetone, insoluble in mineral oil. Slightly soluble in water. 25 ° C in water solubility of 5.9g / 100ml. |
|
Definition |
ChEBI: A triglyceride obtained by acetylation of the three hydroxy groups of glycerol. It has fungistatic properties (based on release of acetic acid) and has been used in the topical treatment of minor dermatophyte infections. |
|
Taste threshold values |
Sweet and creamy with an oily mouthfeel. |
|
General Description |
Triacetin is a triester of glycerin and acetic acid that occurs naturally in papaya. It is mainly used as a synthetic flavoring agent in ice-creams, nonalcoholic beverages and baked goods. |
InChI:InChI=1/C9H14O6/c1-6(10)13-4-9(15-8(3)12)5-14-7(2)11/h9H,4-5H2,1-3H3
Esterification of glycerol with acetic a...
Ru complexes have been utilized as catal...
Triacetin (or glycerol triacetate) was o...
Stable isotope kinetic studies play an i...
The efficient and selective acetylation ...
Carbon-based acid catalysts with porous ...
Acetylation of glycerol with acetic acid...
A series of highly active, selective, an...
SO42-/SnO2 was employed for the acylatio...
(Figure Presented) It comes out in the w...
-
Mono-, di- and triacetin are three glyce...
Novel double-SO3H functionalized ionic l...
Different solid acid catalysts, sulfonic...
Continuous esterification of glycerol wi...
The catalysts dibutyltin dichloride (Bu2...
A convenient, efficient and fast acetyla...
-
The simultaneous synthesis of butyric ac...
Novel excellent hydrophobic POSS-derived...
Supported iron oxide nanoparticles on si...
Rapid and efficient acetylation of alcoh...
-
-
Acyclic and cyclic acetates of various a...
Acetylation of various alcohols and phen...
Herein, we describe the guanidine-promot...
Hierarchical mordenite catalysts were pr...
Cerium(III) triflate is a powerful catal...
HY Zeolite is found to be a versatile ca...
An easy acetylation of alcohols and phen...
The Langmuir–Hinshelwood–Hougen–Watson (...
Antimony trichloride has been found to b...
The esterification of carboxylic acids w...
In the present study, a sulphated silice...
Yttria-zirconia based strong Lewis acid ...
Embelin, a major constituent of Embelia ...
The extracellular polysaccharide from Kl...
Current industrial methods of biodiesel ...
An amide/iminium zwitterion catalyst has...
Zirconium incorporated three-dimensional...
Solid acid catalyst plays a crucial role...
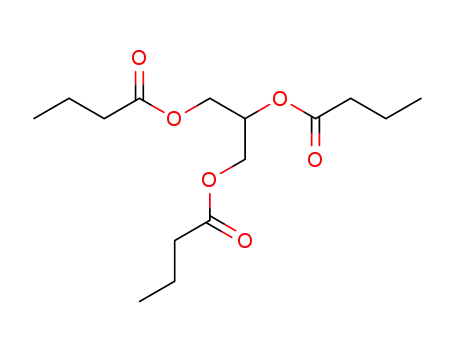
tributyrin

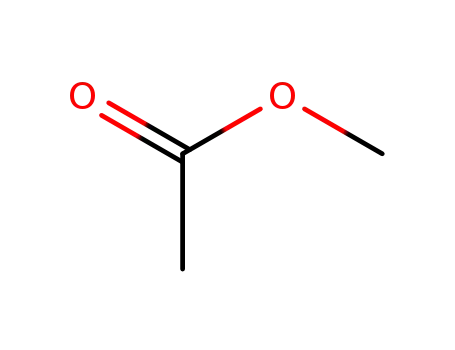
acetic acid methyl ester

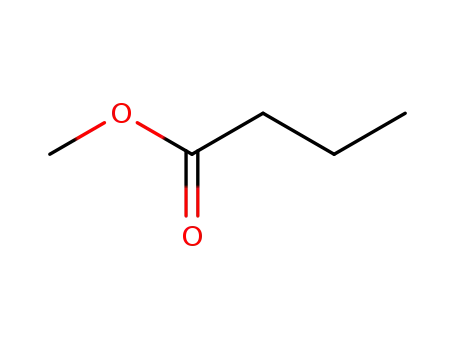
butanoic acid methyl ester

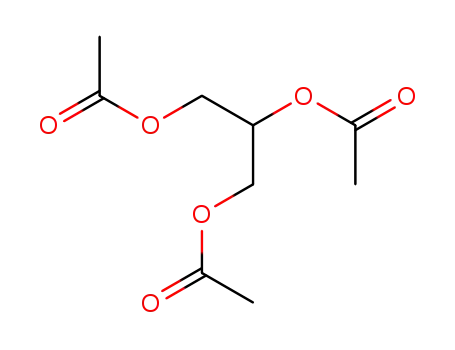
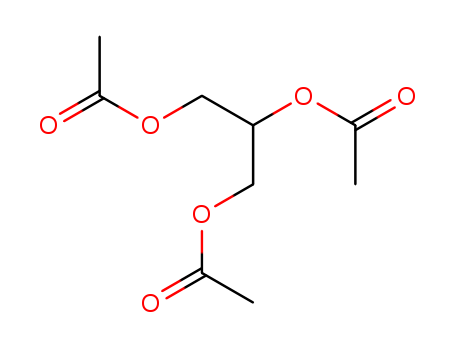
triacetylglycerol

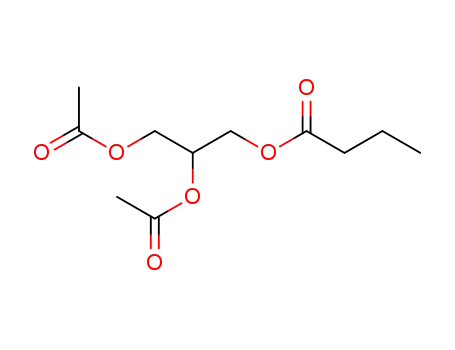
3-Butyryl-1,2-diacetyl-sn-glycerin
| Conditions | Yield |
|---|---|
|
With sodium methylate; at 60 ℃; for 0.2h;
|
68 %Chromat. 25 %Chromat. |

tributyrin


acetic acid methyl ester


butanoic acid methyl ester


triacetylglycerol


3-Butyryl-1,2-diacetyl-sn-glycerin

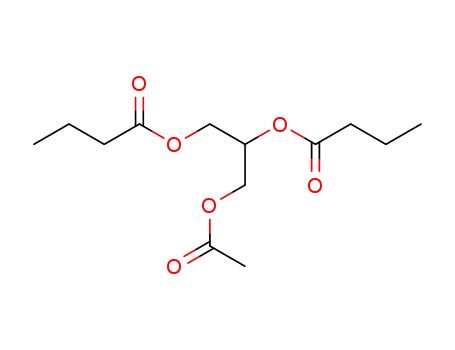
Aceto-dibutyrin
| Conditions | Yield |
|---|---|
|
With trifluorormethanesulfonic acid; acetic acid; at 130 ℃; for 20h;
|
69 %Chromat. 25 %Chromat. 5 %Chromat. |
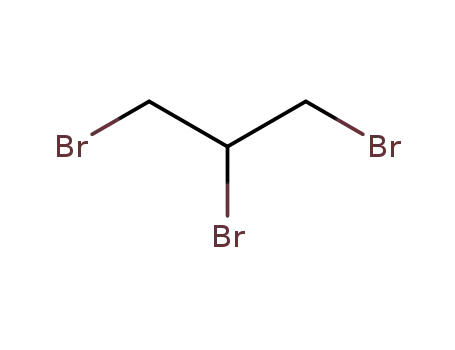
1,2,3-tribromopropane
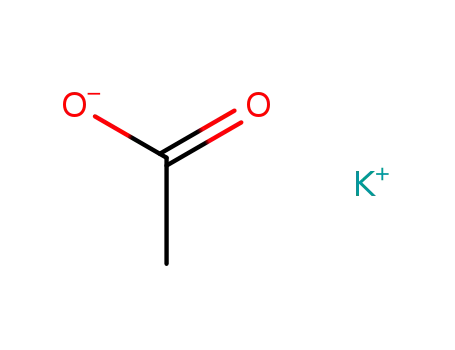
potassium acetate
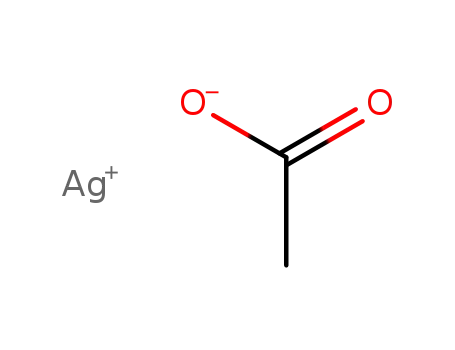
silver(I) acetate
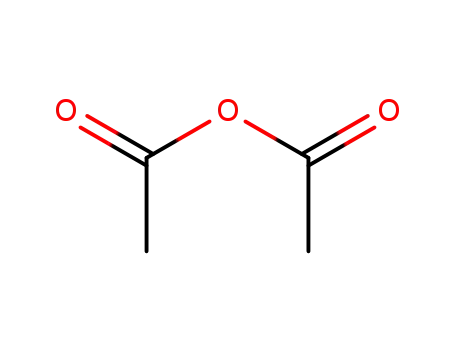
acetic anhydride

acetic acid methyl ester
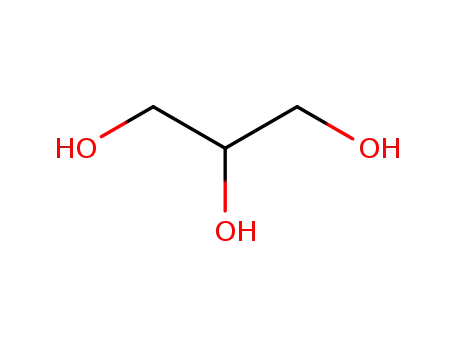
glycerol
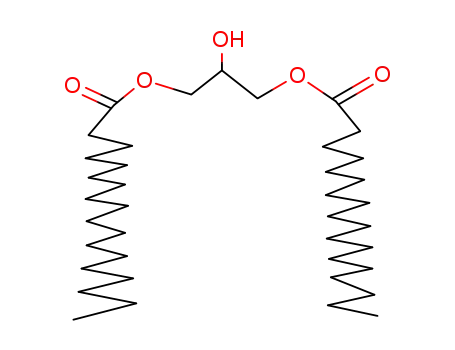
1,3-distearoylglycerol
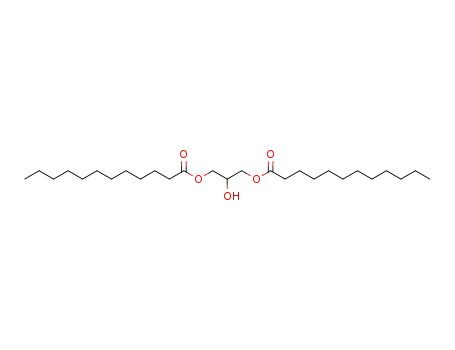
1,3-dilauroylglycerol
