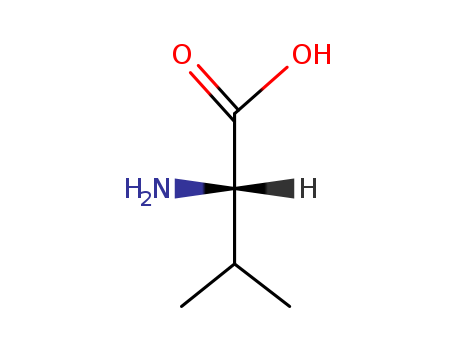
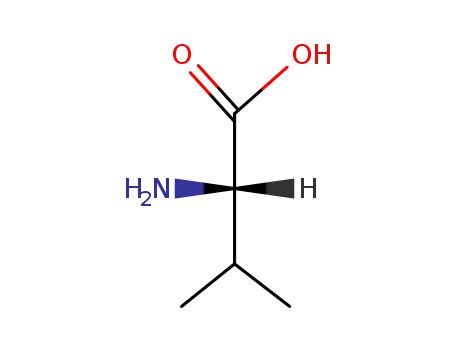
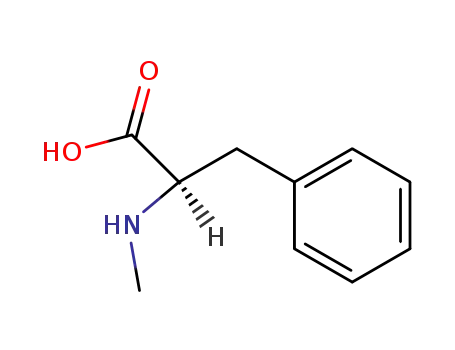
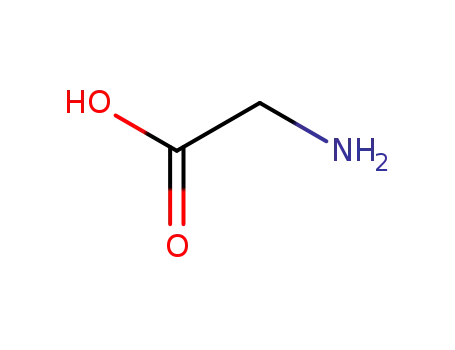
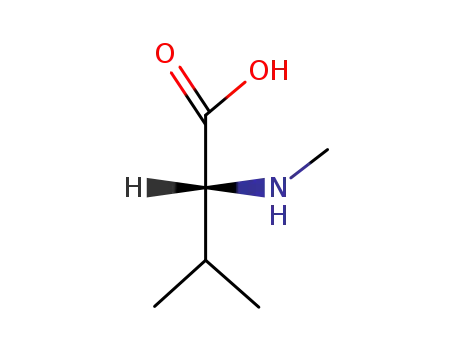
Factory supply good quality L-Valine 72-18-4 with stock
- Molecular Formula:C5H11NO2
- Molecular Weight:117.148
- Appearance/Colour:White crystalline powder
- Melting Point:295-300 °C (subl.)(lit.)
- Refractive Index:28 ° (C=8, HCl)
- Boiling Point:213.642 °C at 760 mmHg
- PKA:2.37±0.10(Predicted)
- Flash Point:83.008 °C
- PSA:63.32000
- Density:1.064 g/cm3
- LogP:0.75460
L-Valine(Cas 72-18-4) Usage
|
Industrial Applications
|
Description: Valine (L-valine) is one of the three branched-chain amino acids (BCAAs) widely applied in various industrial fields such as pharmaceuticals, cosmetics, food, and feed.
Importance in Animal Feed: Valine is among the four most limiting amino acids in low-crude protein diets for piglets and broiler chicks, and its deficiency negatively impacts animal growth performance.
Increasing Demand: There has been a significant increase in demand for Valine in animal feed additives, driving interest in more efficient and economical production methods.
|
|
Biological Importance
|
Role in Muscle Tissue Recovery and Repair: Valine aids in muscle tissue recovery and repair, as well as increasing energy and endurance.
Medicinal Qualities: Valine possesses medicinal qualities for lowering elevated blood sugar levels and enhancing growth hormone production.
Chemical Structure: Valine contains an amino group, a carboxyl group, and a sidechain isopropyl group.
|
|
Medical and Nutritional Uses
|
Inhibition of Tissue Protein Degradation: Valine can inhibit the degradation of tissue proteins, improving the carcass quality of livestock and poultry.
Effects on Immune Function: Valine affects immune function by influencing the development of immune organs and the synthesis of immunoglobulins.
Market Demand: The increasing demand for L-valine in the market highlights the need for cost-effective production methods.
|
|
Production Methods
|
Microbial Fermentation: Currently, microbial fermentation is the primary method for producing L-valine, with mutagenesis and metabolic engineering strategies employed to enhance production.
Challenges: Despite progress in modification strategies, challenges remain in constructing more efficient industrial strains for L-valine production.
Metabolic Strategies: Various metabolic strategies have been used to increase L-valine titers, including mutagenesis, pathway modification, and enhancing extracellular transport.
|
|
General Description
|
Here is a concise paragraph summarizing L-Valine based on the provided abstracts: **L-Valine** is a branched-chain essential amino acid with the chemical formula (2S)-2-amino-3-methylbutanoic acid. It serves as a key building block in peptide synthesis, as demonstrated in studies involving stable peptide helices, enzyme inhibitors (e.g., human leukocyte elastase), and chiral catalysts for asymmetric reactions. Its incorporation into peptides influences secondary structures, such as 310-helices, and enhances stereoselectivity in organocatalysis. Additionally, L-Valine derivatives are utilized in prodrug design (e.g., naproxen conjugates) and chemoenzymatic synthesis (e.g., 4-amino-2-hydroxy acids), highlighting its versatility in medicinal chemistry and biocatalysis. *(Note: The paragraph synthesizes relevant findings while excluding direct references to the literature itself.)*
|
InChI:InChI:1S/C5H11NO2/c1-3(2)4(6)5(7)8/h3-4H,6H2,1-2H3,(H,7,8)
72-18-4 Relevant articles
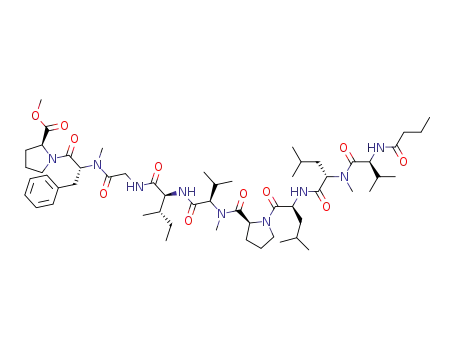
Cyclic Tetrapeptides with Synergistic Antifungal Activity from the Fungus Aspergillus westerdijkiae Using LC-MS/MS-Based Molecular Networking
Chen, Baosong,Dai, Huanqin,Han, Junjie,Li, Erwei,Liu, Hongwei,Lyu, Zhitang,Song, Fuhang,Sun, Jingzu,Wang, Hanying,Wang, Tao,Wang, Wenzhao,Zhang, Rui
, (2022/02/17)
Fungal natural products play a prominent...
Structures and antitumor activities of ten new and twenty known surfactins from the deep-sea bacterium Limimaricola sp. SCSIO 53532
Chen, Min,Chen, Rouwen,Ding, Wenping,Li, Yanqun,Tian, Xinpeng,Yin, Hao,Zhang, Si
, (2022/01/11)
Surfactins are natural biosurfactants wi...
Enhanced carboxypeptidase efficacies and differentiation of peptide epimers
Sung, Yu-Sheng,Putman, Joshua,Du, Siqi,Armstrong, Daniel W.
, (2022/01/29)
Carboxypeptidases enzymatically cleave t...
Inherently chiral dialkyloxy-calix[4]arene acetic acids as enantiodiscriminating additives for high-performance liquid chromatography separation of d,l-amino acids
Kalchenko, Olga I.,Trybrat, Oleksandr O.,Yesypenko, Oleksandr A.,Dyakonenko, Viktoriya V.,Shishkina, Svitlana V.,Kalchenko, Vitali I.
, p. 722 - 730 (2021/08/26)
Inherently chiral dialkyloxy-calix[4]are...
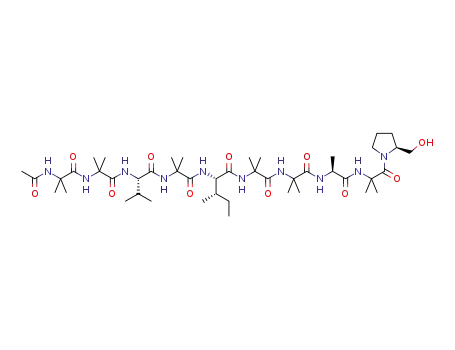
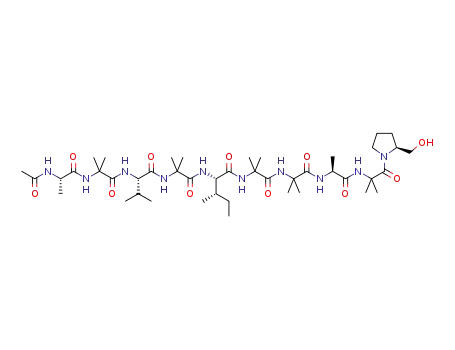
72-18-4 Process route
-
- 56-41-7,25191-17-7,18875-37-1
L-alanin
-
- 72-18-4,25609-85-2,7004-03-7,921-10-8
L-valine
-
- 1783-96-6,25608-40-6,27881-03-4,32505-46-7,52526-39-3
(2R)-aspartic acid
Conditions
| Conditions |
Yield |
|
With hydrogenchloride; water; at 120 ℃; for 14h;
|
|
-
- 72-18-4,25609-85-2,7004-03-7,921-10-8
L-valine
-
- 61-90-5,21675-61-6,25248-98-0,70-45-1
L-leucine
-
- 73-32-5,959215-79-3,18875-42-8
L-isoleucine
-
- 3060-46-6
N-methyl-L-leucine
-
- 56564-52-4
N-methyl-D-phenylalanine
-
- 56-40-6,18875-39-3,25718-94-9
glycine
-
- 88930-14-7
(-)-α-methylamino-β-methylbutyric acid
-
- 147-85-3,25191-13-3,37159-97-0,4305-67-3,4607-28-7
L-proline
Conditions
| Conditions |
Yield |
|
With hydrogenchloride; In water; at 110 ℃; for 24h;
|
|
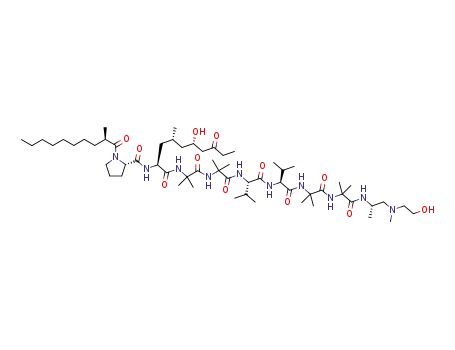
72-18-4 Upstream products
-
1236074-95-5
trichoderin A1
-
1236074-96-6
trichoderin B
-
1169571-12-3
aspereline A
-
1169571-13-4
aspereline B
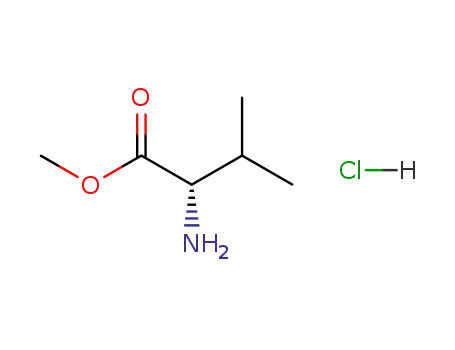
72-18-4 Downstream products
-
6306-52-1
L-valine methylester hydrochloride
-
6306-54-3
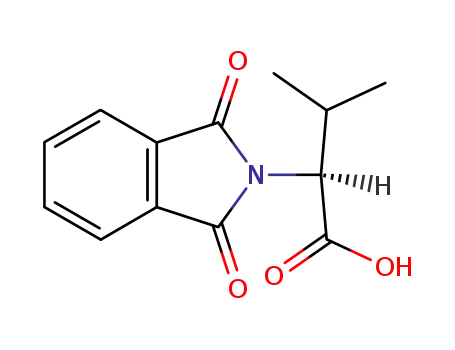
N-phthaloyl L-valine
-
10003-64-2
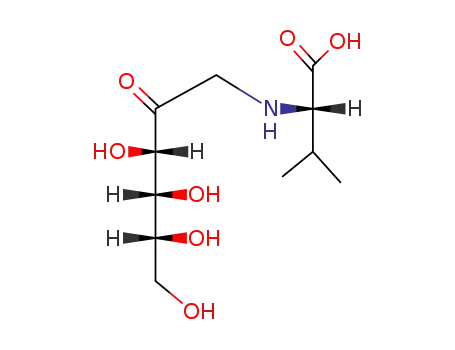
N-(1-deoxy-D-fructos-1-yl)-L-valine
-
349-00-8
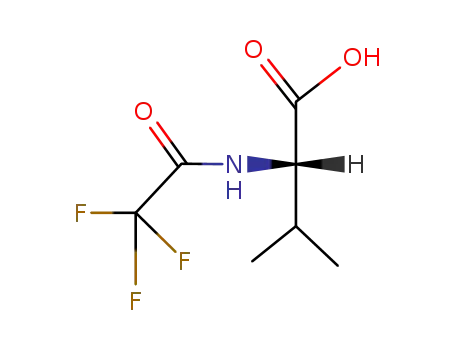
N-trifluoroacetyl-L-valine