
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Chemical Description |
Guanidine hydrochloride, urea, and thiourea are reagents used in the synthesis of dihydropyrimido[4,5-c]pyridazine-3-carboxanilide derivatives. |
|
Protein Denaturation |
Guanidine Hydrochloride (GdnHCl) is a better denaturant than urea.It should normally give higher and lower values for m and for Cm, respectively, but identical deltaG° values. Since m values in both denaturants can be predicted/estimated from the size of the protein (Myers et al., 1995) and delatG° should not change, yes it is possible-in principle-to predict Cm in one denaturant, knowing its value in the other. but to have a general correlation co-efficient is almost impossible as every protein has unique folding. Though such co-relation may be found for a special protein fold, but what purpose it may serve apart from giving a tentative idea that GdnHCl or urea may be a better denaturant! If you are looking for even better denaturant than Gdn HCl then use GdnSCN, it is better and more effective |
|
Drug intermediates |
Guanidine hydrochloride drug used primarily as an intermediate in the manufacture of sulfadiazine, an important raw material sulfamethyldiazine, sulfamethazine and folic acid. Guanidine hydrochloride (or guanidine nitrate) and ethyl cyanoacetate reaction, cyclization of 2,4-diamino-6-hydroxy pyrimidine, folic acid for the synthesis of antianemic. Furthermore guanidine hydrochloride also used synthetic anti-static agent. |
|
Preparation |
In Dicyanodiamide and ammonium (Ammonium chloride) as raw material, melt reaction at 170-230 ℃, guanidine hydrochloride obtained crude, refined products. |
|
Reference quality standards |
Name of Project Specifications Pharmaceutical grade industrial grade Appearance white crystal; white crystal Content > 99% 99.5% Ammonium <0.3% 0.5% Water Insoluble substance <0.1% 0.1% Moisture <0.3% 0.3% Ash <0.05% 0.05% Water-soluble test qualified; qualified Melting range °C 184-188; 182-188 Absorbance (UV wavelength 260NM) <=0.04 Absorbance (UV wavelength 280NM) <=0.02 PH value (4% aqueous solution) 6.4 ± 0.2 6.4 ± 0.2 |
|
Hazard |
Toxic by ingestion, evolves hydrogen chloride fumes on heating. |
|
Biochem/physiol Actions |
Guanidine hydrochloride (GdnHCl) is involved in conformational changes and loss of enzyme activity due to inactivation of the enzyme. It is involved in the loss of activity of alkaline phosphatase (ALPase) enzyme in Haliotis diversicolor. GdnHCl functions by hampering the activity of heat shock protein 104 (Hsp104) adenosine triphosphatase (ATPase) in vivo. It can also inactivate enzymes like papain and aminocyclase. Higher concentrations of GdnHCl leads to viral inactivation of herpes simplex virus 1 (HSV-1). |
|
Purification Methods |
Crystallise the hydrochloride from hot methanol by chilling to about -10o, with vigorous stirring. The fine crystals are filtered through fritted glass, washed with cold (-10o) methanol, dried at 50o under vacuum for 5hours. (The product is purer than that obtained by crystallisation at room temperature from methanol by adding large amounts of diethyl ether.) [Kolthoff et al. J Am Chem Soc 79 5102 1957, Beilstein 3 H 86, 3 II 71, 3 III 160, 3 IV 150.] |
|
description |
Guanidine hydrochloride is a strong organic base existing primarily as guanidium ions at physiological pH. It is found in the urine as a normal product of protein metabolism. It is also used in laboratory research as a protein denaturant.It is also used in the treatment of myasthenia and as a fluorescent probe in HPLC. Guanidine Hydrochloride is the hydrochloride salt form of guanidine, a strong basic compound with parasympathomimetic activity. Guanidine hydrochloride enhances the release of acetylcholine following a nerve impulse and potentiates acetylcholine actions on muscarinic and nicotinic receptors. It also appears to slow the rates of depolarization and repolarization of muscle cell membranes. Guanidine Hydrochloride is a powerful, chaotropic agent which is widely used for purification of proteins and nucleic acids. Guanidine Hydrochloride is useful for denaturation and refolding of proteins as well as in the recovery of periplasmic proteins and isolation of RNA. At higher concentrations, this strong denaturant has been reported to solubilize denatured insoluble proteins such as inclusion bodies. When utilized at lower concentrations, Guanidine Hydrochloride is noted to be useful in prompting the refolding of denatured proteins. Guanidine Hydrochloride is also been reported to allow return of enzymatic activity in protocols using Guanidine Hydrochloride as a first step. Guanidine Hydrochloride is an inhibitor of RNase. Guanidine hydrochloride is used for purification of proteins and nucleic acids. It can be used as a medicine, organic synthesis intermediate and is used in dyes. It acts as an intermediate in the preparation of sulfadiazine, which is an important raw material for sulfamethyldiazine, sulfamethazine and folic acid. It is utilized in the synthesis of 2-amino-pyrimidine, 2-amino-6-methyl-pyrimidine and 2-amino-4,6-dimethyl-pyrimidine. Also used in RNA isolation to dissociate nucleoproteins and inhibit RNase. |
|
Definition |
ChEBI: Guanidine Hydrochloride is an aminocarboxamidine, the parent compound of the guanidines. It is a strong organic base existing primarily as guanidium ions at physiological pH. It is found in the urine as a normal product of protein metabolism. |
|
Application |
Guanidine hydrochloride can be used as pharmaceuticals, pesticides, dyes and other organic synthesis intermediates. It can be used to synthesize 2-aminopyrimidine, 2-amino-6-methylpyrimidine, 2-amino-4,6-dimethylpyrimidine, and is used for the manufacture of sulfadiazine, sulfamethazine, sulfamethazine and other sulfa drugs. Intermediate. Guanidine hydrochloride (or guanidine nitrate) reacts with ethyl cyanoacetate to form 2,4-diamino-6-hydroxypyrimidine, which is used to synthesize the anti-anemia drug folic acid. It can also be used as an antistatic agent for synthetic fibers. Can also be used for protein denaturants. As a strong denaturant in experiments for extracting total cellular RNA[3]. The guanidine hydrochloride solution can dissolve the protein, leading to the destruction of the cell structure, the destruction of the secondary structure of the nucleoprotein, and the dissociation from the nucleic acid. In addition, the RNase can be inactivated by reducing agents such as guanidine hydrochloride. |
|
General Description |
Guanidine hydrochloride (GdnHCl) is a well-known protein and enzyme denaturant. It is a small molecule and hydroscopic in nature. |
InChI:InChI=1/CH5N3.ClH/c2-1(3)4;/h(H5,2,3,4);1H/p+1
Reductions under neutral conditions of m...
Guanidinium trinitromethanide monohydrat...
Phase change materials (PCMs), which mel...
The present invention pertains to a meth...
Herein we report on our systematic inves...
The present invention relates to humaniz...
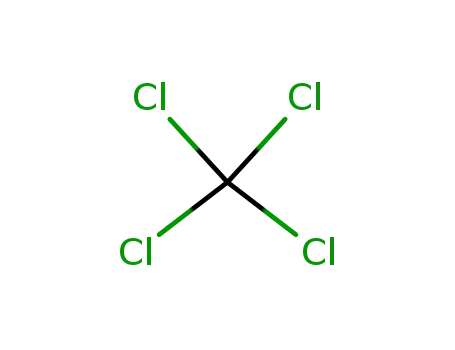
tetrachloromethane

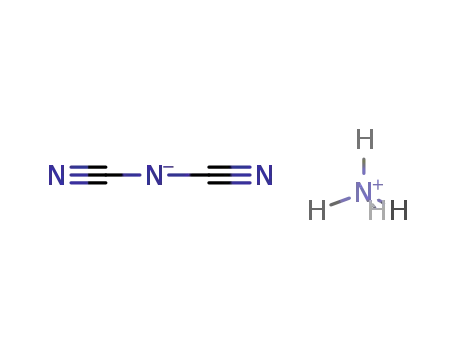
ammonium dicyanamide

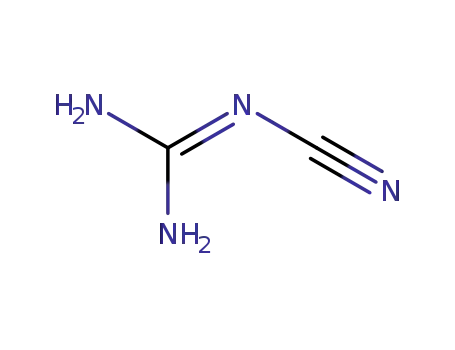
dicyandiamide

hexachloroethane

ammonium chloride

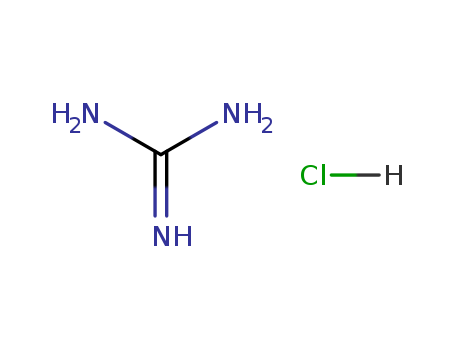
guanidine hydrochloride
| Conditions | Yield |
|---|---|
|
With ammonia; In tetrachloromethane; other Radiation; γ-radiation of NH3 soln. in CCl4 at 25°C; mechanism discussed; further products and further intermediates;;
|
ammonium bicarbonate


guanidine hydrochloride
| Conditions | Yield |
|---|---|
|
|
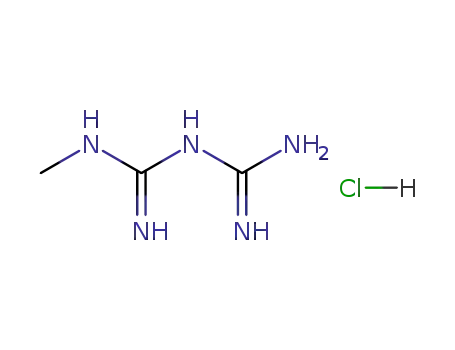
N1-methyl biguanide hydrochloride
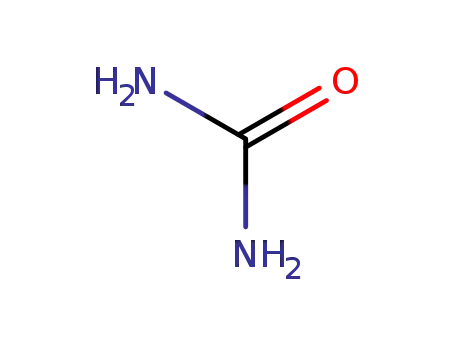
urea
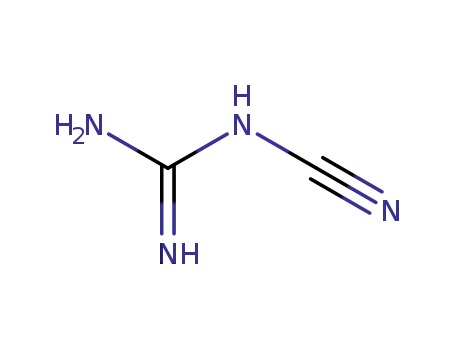
N-Cyanoguanidine

tetrachloromethane
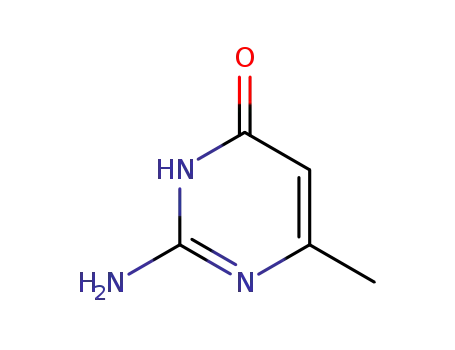
2-amino-6-methyl-3H-pyrimidin-4-one
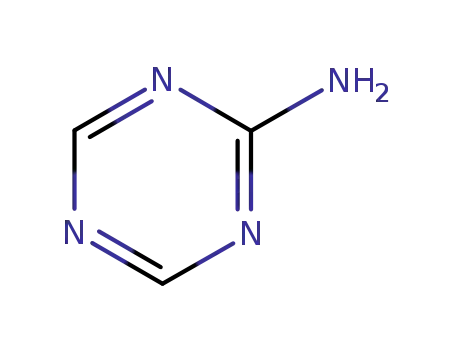
2-amino-1,3,5-triazine
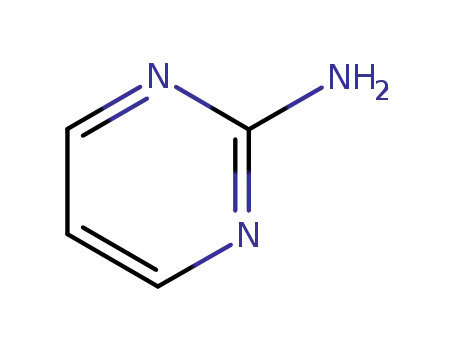
2-aminopyrimidine
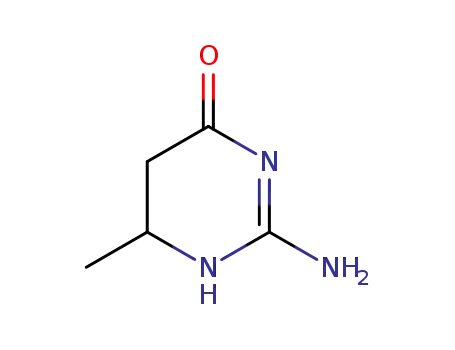
2-amino-6-methyl-5,6-dihydro-3H-pyrimidin-4-one
