
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Product Features |
Carbohydrazide is a white crystalline thin and short columnar crystal or white powder at room temperature. It is insoluble in alcohol, easily soluble in water with dissolution absorbing heat. It is insoluble in alcohol, ether, and benzene. Owing to that, it is a kind of derivative of hydrazine and thus having strong reduction ability. It is non-toxic, and can replace hydrazine and oximes. It has a broad range of application in industry. For example, it can be used as the oxygen scavenging agent of boiler water in the field of water treatment and is regarded as most advanced materials for oxygen scavenging of boiler water. It has a low toxicity and high melting point with its deoxidizing efficiency being far greater than the current materials used and is a idea product for both safety and environmental protection; it can also be used as a rocket propellant components; moreover, owing to that its hydrogen atoms attached to the nitrogen atom is easily substituted by other groups, it can be used as the cross-linking agents of elastic fibers in the textile field, the formaldehyde scavenger, as well as the antioxidant of carotene pigment. In addition, adding an appropriate amount of carbohydrazide to the phenol fungicides containing can play a role on preventing discoloration and rancidity. As a chemical raw material and chemical industry intermediates, it is widely used in medicine, herbicides, plant growth regulators, dyes and other industries. |
|
Oxygen scavenger of the boiler water |
When acting as the oxygen scavenger of boiler water, carbohydrazide may be directly added into the water while its aqueous solution can also be used. The usage amount of carbohydrazide for scavenging 1mol O2 is 0.5mol, and should be appropriately in excess. The proper temperature range is 87.8-176.7 ℃. The optimal time for applying carbohydrazide is after the thermal scavenging of oxygen. The reaction of oxygen and carbohydrazide is as follows: CON4H6 + 2O2 = 2N2 + 3H2O + CO2 |
|
Reference quality standards |
Item Index Appearance: fine white crystals or white short columnar crystals Purity% ≥98.0% Free hydrazine ≤250.0mg/L Loss of weight by drying ≤0.2% PH (12% aqueous solution) 8.45 ± 1.25 |
|
Toxicity grading |
poisoning |
|
Acute toxicity |
Intraperitoneal-mouse LD50: 167 mg/kg; Intravenous-Mouse LD50: 120 mg/kg. |
|
Explosive and hazardous characteristics |
it is explosive upon heating; it can generate explosive diazide compound upon reaction with nitrous acid. |
|
Flammability and hazard characteristics |
thermal decomposition into toxic nitric oxide gas. |
|
Storage characteristics |
Treasury: ventilation, low-temperature and drying; it should be stored separately from strong acids and strong oxidants. |
|
Extinguishing agent |
Water, carbon dioxide, dry powder, foam. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Poison by intravenous and intraperitoneal routes. Reacts with nitrous acid to form the explosive carbonic dazide. When heated to decomposition it emits toxic fumes of NOx. |
|
Category |
toxic substances |
|
Definition |
ChEBI: A carbohydrazide obtained by formal condensation between hydrazinecarboxylic acid and hydrazine. |
InChI:InChI=1/CH6N4O/c2-4-1(6)5-3/h2-3H2,(H2,4,5,6)
The invention provides a preparation met...
The invention relates to a carbonyl dihy...
The invention relates to a continuous hy...
The invention discloses a method for pre...

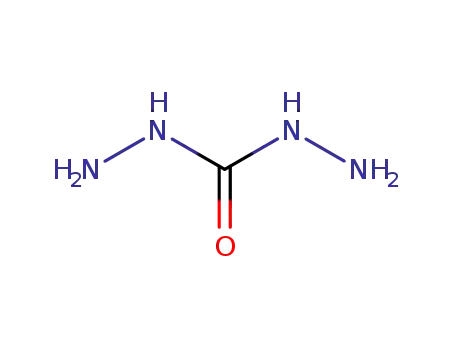
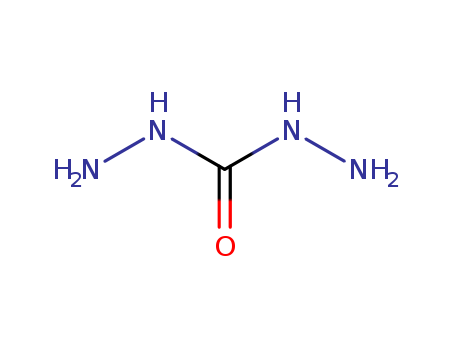
carbonodihydrazide

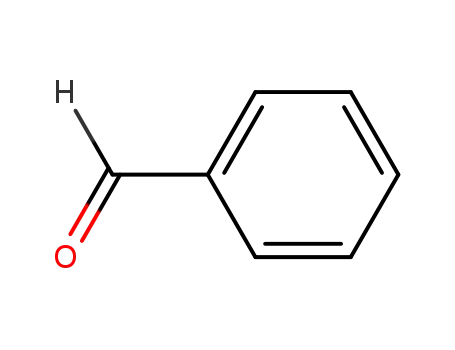
benzaldehyde
| Conditions | Yield |
|---|---|
|
With diluted acid; Nebenprod.2:Hydrazin;
|
N,N',N''-tris-benzylidenamino-guanidine


carbonodihydrazide


benzaldehyde

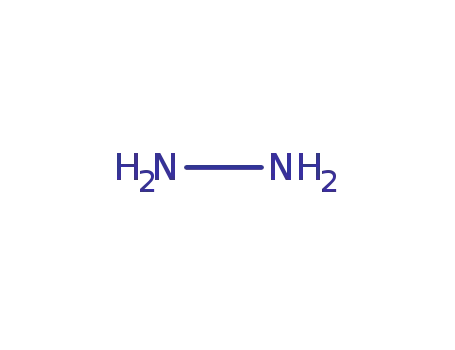
hydrazine
| Conditions | Yield |
|---|---|
|
|
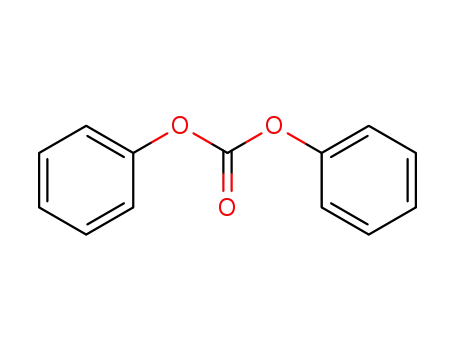
bis(phenyl) carbonate
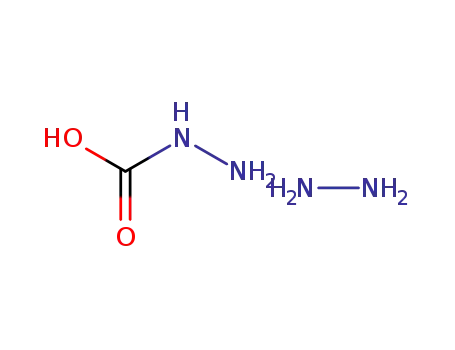
hydrazonium hydrazincarbonate
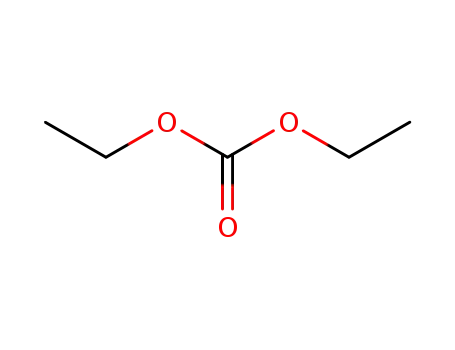
Diethyl carbonate
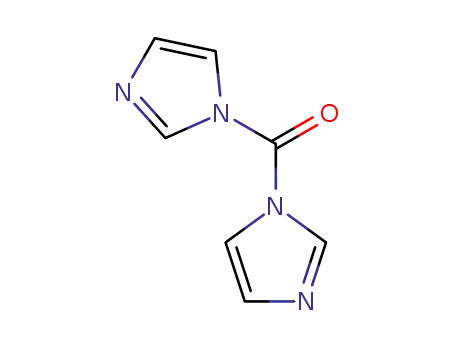
1,1'-carbonyldiimidazole
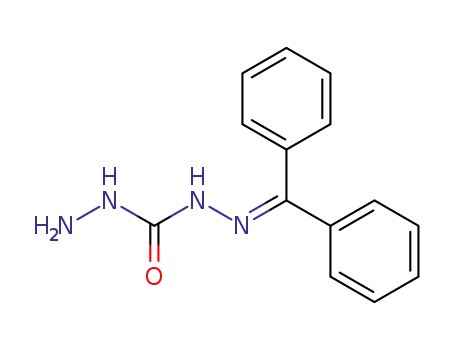
1-benzhydrylidene carbonohydrazide
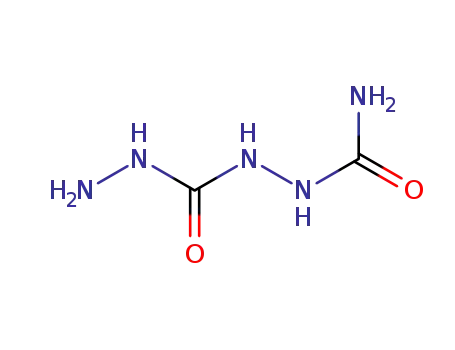
1-Carbamoyl-carbohydrazid
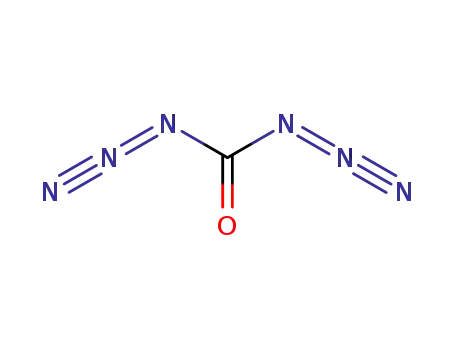
carbonyl azide
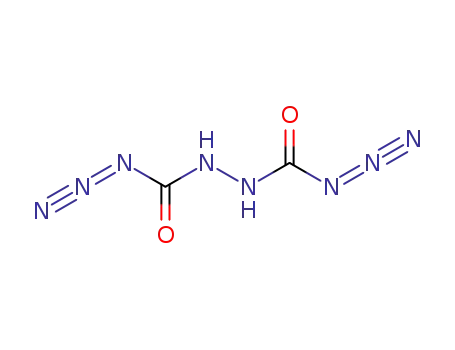
Hydrazodicarbonazid (germ.)=hydrazodicarbonic azide
