
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Synthesis Reference(s) |
Synthesis, p. 470, 1996 DOI: 10.1055/s-1996-4246 |
|
Purification Methods |
Distil 2-naphthaldehyde with steam then crystallise it from water or EtOH. [Beilstein 7 IV 1288.] |
|
Definition |
ChEBI: A naphthaldehyde that is naphthalene substituted by a formyl group at position 2. |
InChI:InChI=1/C11H8O/c12-8-9-5-6-10-3-1-2-4-11(10)7-9/h1-8H
Mo-modified V2O5/TiO2 catalysts were pre...
Heterogeneous nitrogen-doped carbon-inca...
A practical method is disclosed for the ...
We have developed a highly atom efficien...
A highly efficient, site-selective benzy...
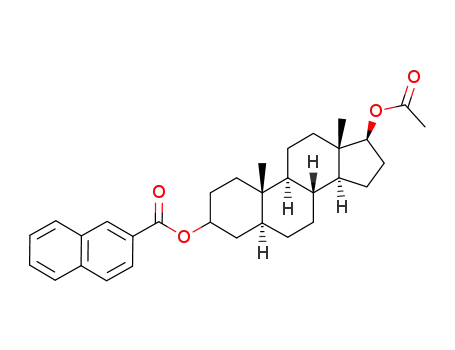
17β-acetoxyandrost-2-en-3-yl 2-naphthoate

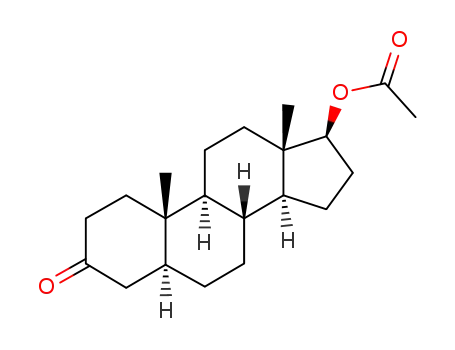
stanolone acetate

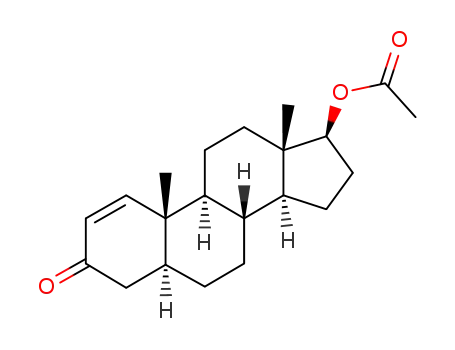
(5α,17β)-3-oxoandrost-1-en-17-yl acetate

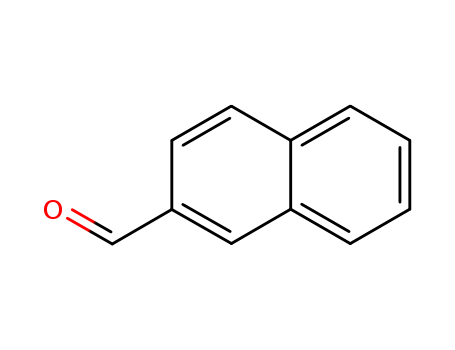
β-naphthaldehyde

![Acetic acid (5S,8R,9S,10S,13S,14S,17S)-10,13-dimethyl-2-(naphthalene-2-carbonyl)-3-oxo-hexadecahydro-cyclopenta[a]phenanthren-17-yl ester](/upload/2025/4/3fb66f59-e042-4baf-9815-17e9a1fa784c.png)
Acetic acid (5S,8R,9S,10S,13S,14S,17S)-10,13-dimethyl-2-(naphthalene-2-carbonyl)-3-oxo-hexadecahydro-cyclopenta[a]phenanthren-17-yl ester
| Conditions | Yield |
|---|---|
|
In hexane; at 0 ℃; Quantum yield; Mechanism; Product distribution; Irradiation; variation of wavelenght, concentration, solvent, irradiation intensity, reaction time;
|
|
|
In cyclohexane; for 2h; Irradiation;
|
15 mg 30 mg 25 mg 250 mg |
5-(2-Naphthoyl)-5H-benzocarbazole

2,3-benzocarbazole


β-naphthaldehyde

6-(2-Naphthoyl)benzocarbazole
| Conditions | Yield |
|---|---|
|
In cyclohexane; at 20 ℃; for 8h; Irradiation;
|
3.5 % Chromat. 1 % Chromat. 0.7% |
2-naphthohydrazide
ethanol
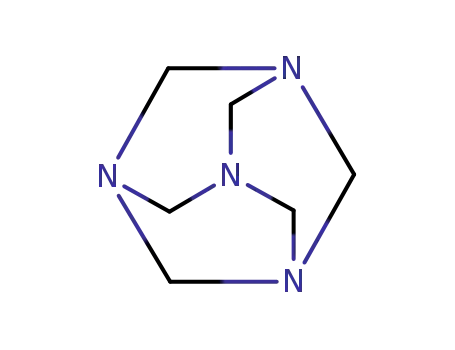
hexamethylenetetramine
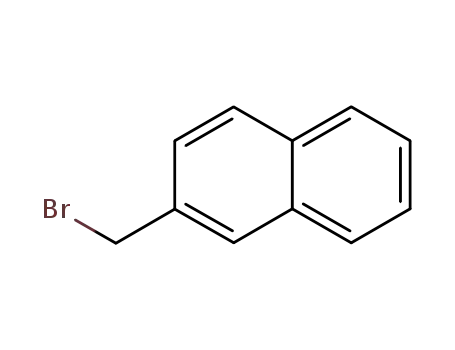
2-bromomethylnaphthyl bromide
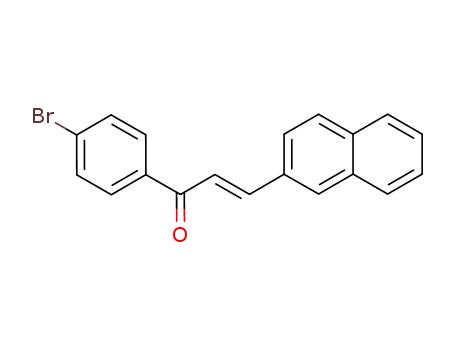
(E)-1-(4-bromophenyl)-3-(naphthalen-2-yl)prop-2-en-1-one
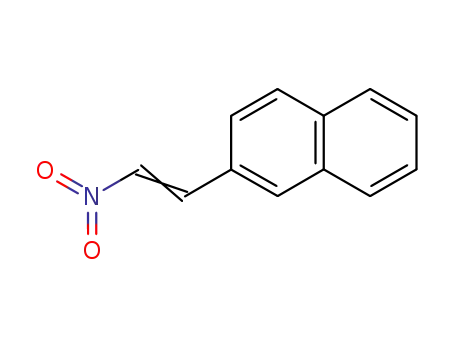
2-(2-nitrovinyl)naphthalene
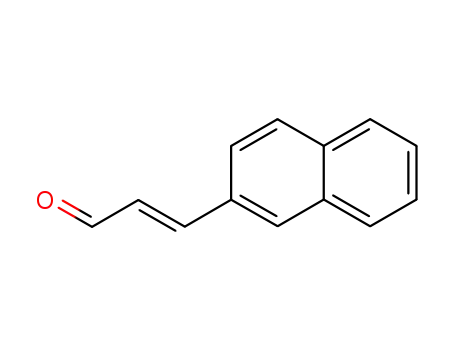
(E)-3-(2-naphthyl)-2-propenal
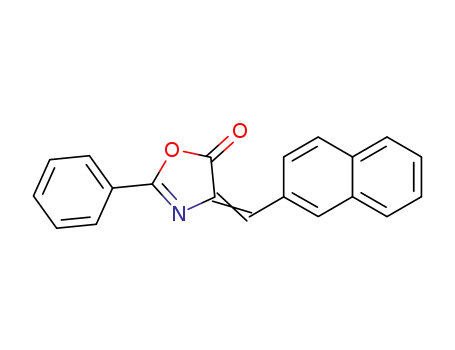
4-(2-naphthylidene)-2-phenyl-5(4H)oxazolone
