
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Production Methods |
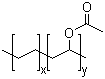
Various molecular weights of random ethylene vinyl acetate copolymers can be obtained by high-pressure radical polymerization, bulk continuous polymerization, or solution polymerization. |
|
Pharmaceutical Applications |
Ethylene vinyl acetate copolymers are used as membranes and backings in laminated transdermal drug delivery systems. They can also be incorporated as components in backings in transdermal systems. Ethylene vinyl acetate copolymers have been shown to be an effective matrix and membrane for the controlled delivery of atenolol triprolidine, and furosemide. The system for the controlled release of atenolol can be further developed using ethylene vinyl acetate copolymers and plasticizers. |
|
Safety |
Ethylene vinyl acetate is mainly used in topical pharmaceutical applications as a membrane or film backing. Generally it is regarded as a relatively nontoxic and nonirritant excipient. |
|
storage |
Ethylene vinyl acetate copolymers are stable under normal conditions and should be stored in a cool, dry place. Films of ethylene vinyl acetate copolymers should be stored at 0–30°C and less than 75% relative humidity. |
|
Incompatibilities |
Ethylene vinyl acetate is incompatible with strong oxidizing agents and bases. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (intrauterine suppository; ophthalmic preparations; periodontal film; transdermal film). Included in nonparenteral medicines licensed in the UK. |
|
Physical properties |
Ethylene vinyl acetate is available as white waxy solids in pellet or powder form. Films are translucent. |
|
Definition |
An elastomer used to improve adhesion properties of hot-melt and pressure-sensitive adhesives, as well as for conversion coatings and thermoplastics. |
|
General Description |
Poly(ethylene-co-vinyl acetate) (PEVA) is a flame-retardant material with good mechanical and physical properties. It is majorly used as an insulating material in wire and cable industry. |
InChI:InChI=1/C4H6O2.C2H4/c1-2-3-4(5)6;1-2/h2H,1,3H2,(H,5,6);1-2H2
