
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Production Methods |
tert-Butyl acetate is prepared from isobutylene reacting with acetic acid in the liquid phase with vanadium pentoxideimpregnated silica as the catalyst and with heat to increase the yield . |
|
Synthesis Reference(s) |
Organic Syntheses, Coll. Vol. 3, p. 142, 1955Synthesis, p. 1015, 1987Tetrahedron Letters, 37, p. 4555, 1996 DOI: 10.1016/0040-4039(96)00902-1 |
|
Air & Water Reactions |
Highly flammable. Insoluble in water. |
|
Reactivity Profile |
tert-Butyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. tert-Butyl acetate is incompatible with the following: Nitrates; strong oxidizers, alkalis & acids . |
|
Hazard |
Flammable, moderate fire risk. Eye and upper respiratory tract irritant. |
|
Health Hazard |
Exposure to tert-butyl acetate causes eye, skin, and respiratory irritation in workers. By analogy with the effects of exposure to similar esters, tert-butyl acetate may act as a CNS depressant at high concentrations. The signs and symptoms of acute exposure to tert-butyl acetate include, but are not limited to, itchy or inflamed eyes and irritation of the nose and upper respiratory tract. Exposures to tert-butyl acetate at high concentrations may cause headache, drowsiness, and other narcotic effects. |
|
Safety Profile |
Poison by inhalation and ingestion. Flammable. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Environmental fate |
Chemical/Physical. Hydrolyzes in water to tert-butyl alcohol and acetic acid. The estimated hydrolysis half-life at 25 °C and pH 7 is 140 yr (Mabey and Mill, 1978). |
|
storage |
tert-Butyl acetate should be stored in a cool, dry, well-ventilated area in tightly sealed containers that are labeled in accordance with regulatory standards. Containers of tert butyl acetate should be protected from physical damage and should be stored separately from nitrates, strong oxidizers, strong acids, strong alkalis, heat, sparks, and open flame. Because containers that formerly contained tert-butyl acetate may still hold product resi dues, they should be handled appropriately |
|
Purification Methods |
Wash the ester with 5% Na2CO3 solution, then saturated aqueous CaCl2, dry with CaSO4 and distil it. [McClosky et al. Org Synth Coll Vol IV 263 1963, Mangia et al. Org Prep Proc Int 18 13 1986, Beilstein 2 IV 151.] |
|
Precautions |
If exposures to tert-butyl acetate or a solution in work areas gets into the eyes, immediately fl ush the eyes with large amounts of water for a minimum of 15 min, lifting the lower and upper lids occasionally. Always use safety goggles or eye protection in combination with breathing protection. |
|
Physical properties |
Colorless liquid with a fruity odor. Odor threshold concentration in water is 4 ppm (CHRIS, 1994). Nagata and Takeuchi (1990) reported an odor threshold concentration of 71 ppbv. |
|
General Description |
Tert-butyl acetate is a colorless liquid with a mild odor. Floats on water. Produces irritating vapor. (USCG, 1999) |
InChI:InChI=1/C6H12O2/c1-5(7)8-6(2,3)4/h1-4H3
A series of solid acid catalysts were sy...
Tert-butyl N-aryl sulfinamoyl acetates a...
We report the dehydrogenative synthesis ...
Abstract: Cost-effective, efficient and ...
A suitable, expeditious and well-organiz...
Baeyer-Villiger monooxygenases (BVMOs) a...
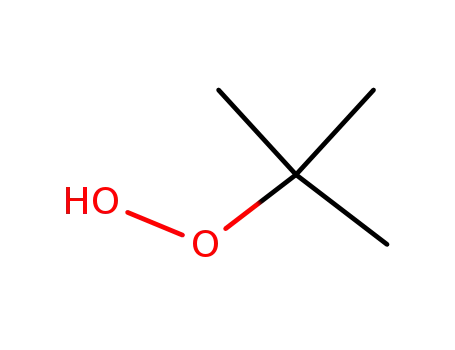
tert.-butylhydroperoxide

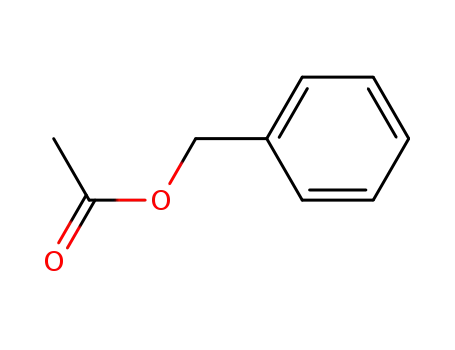
Benzyl acetate

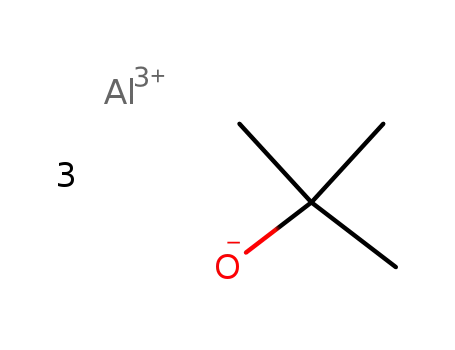
aluminum tri-tert-butoxide

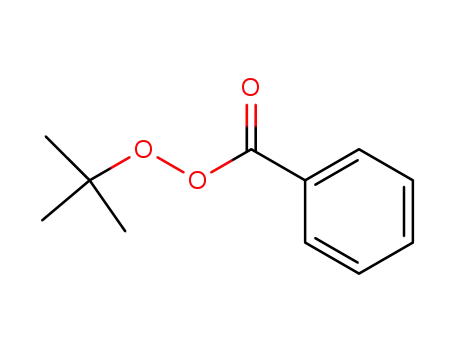
tert-Butyl peroxybenzoate

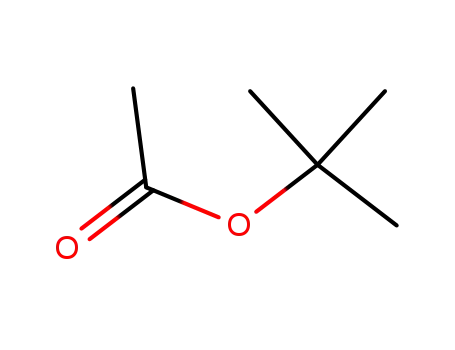
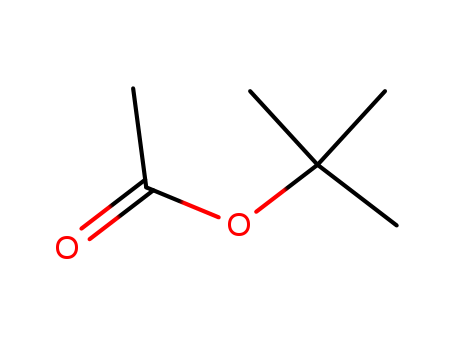
acetic acid tert-butyl ester

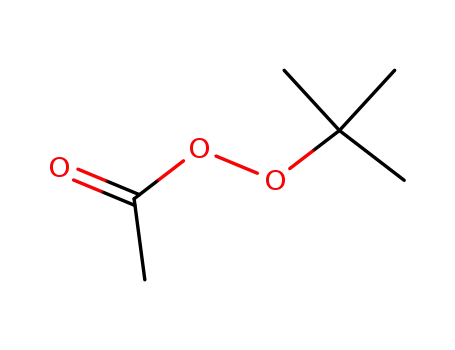
tert-butyl peroxyacetate

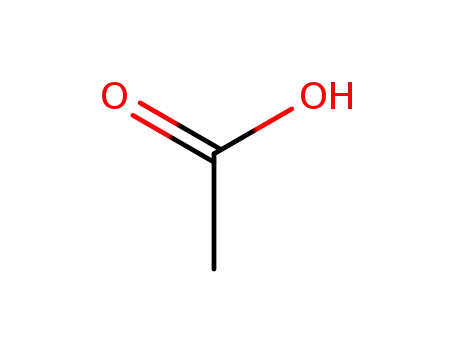
acetic acid

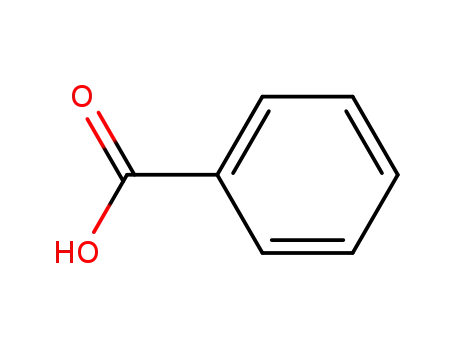
benzoic acid

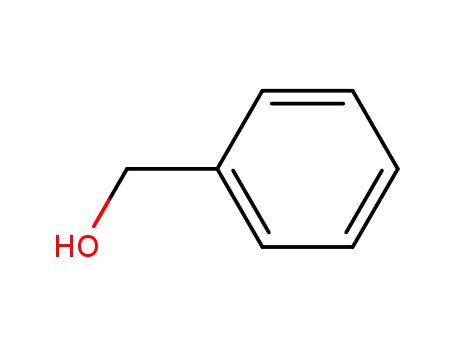
benzyl alcohol
| Conditions | Yield |
|---|---|
|
In
benzene;
at 20 ℃;
for 3h;
Product distribution;
Mechanism;
|
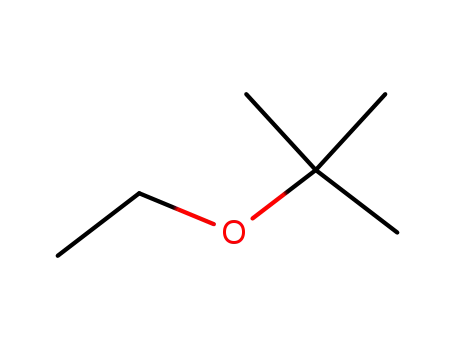
Ethyl tert-butyl ether

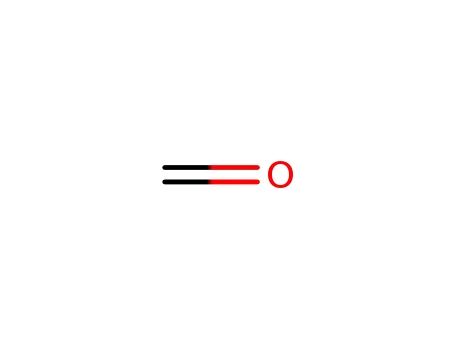
formaldehyd


acetic acid tert-butyl ester

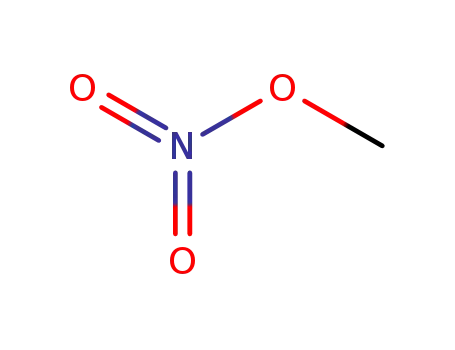
methyl nitrate

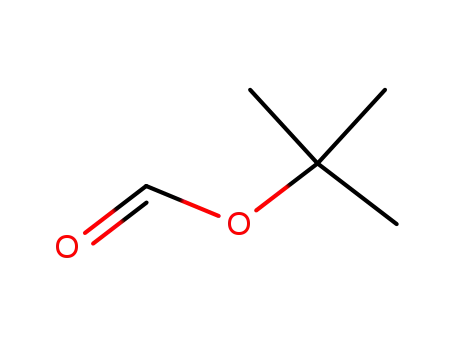
tert-butyl formate
| Conditions | Yield |
|---|---|
|
With
nitrate radical;
In
gas;
at -16.1 - 89.9 ℃;
under 750.06 Torr;
Mechanism;
Kinetics;
|
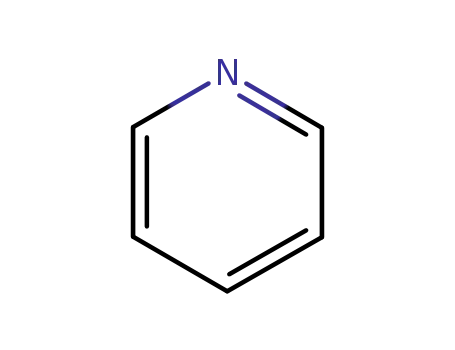
pyridine
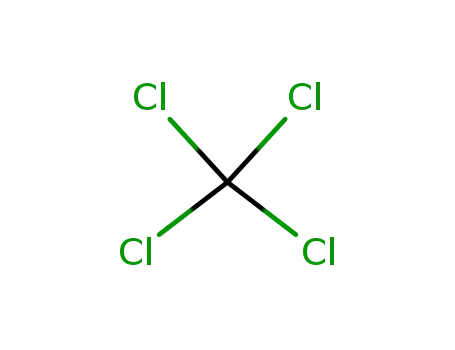
tetrachloromethane
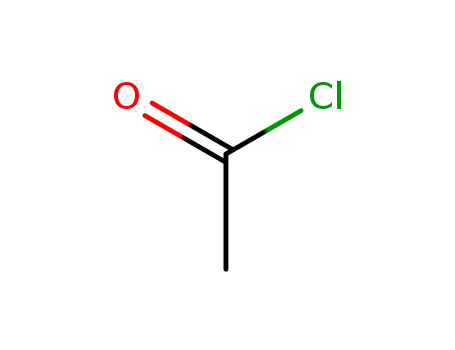
acetyl chloride
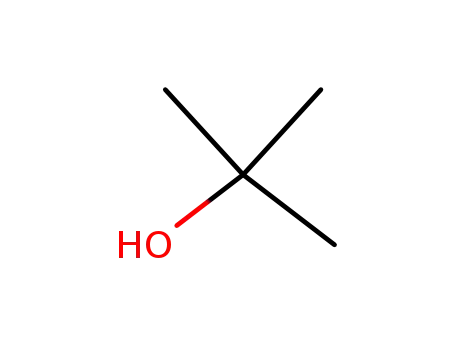
tert-butyl alcohol
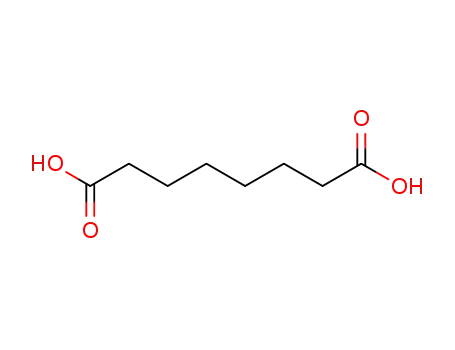
octane-1,8-dioic acid
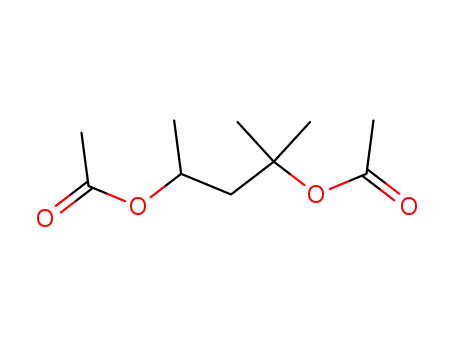
2,4-diacetoxy-2-methylpentan
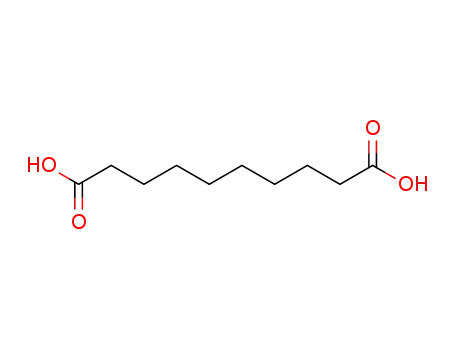
1,10-decanedioic acid
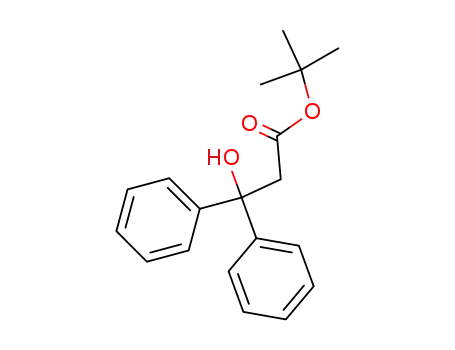
3-hydroxy-3,3-diphenyl-propionic acid tert-butyl ester
