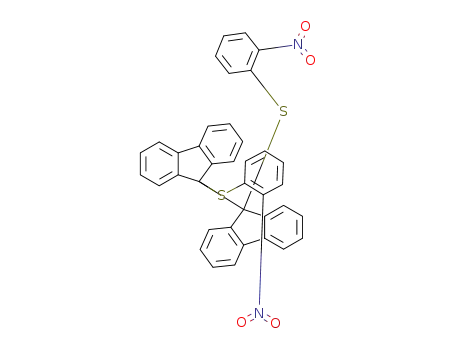
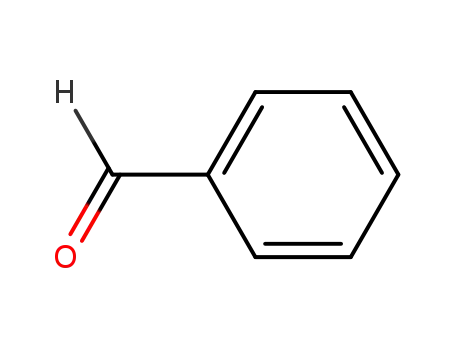
Fulvalene Derivatives Containing a Tetrabenzofluorene Unit: New Nonplanar Fulvalenes with High Electron Affinity
Yamada, Kenta,Shibamoto, Hiroshi,Tanigawa, Yusuke,Ishikawa, Hiroyuki,Nishida, Jun-Ichi,Kitamura, Chitoshi,Kurata, Hiroyuki,Kawase, Takeshi
, p. 2085 - 2090 (2016)
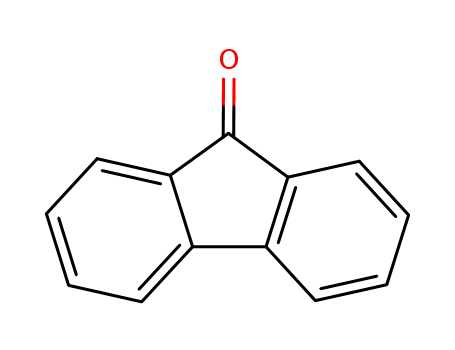
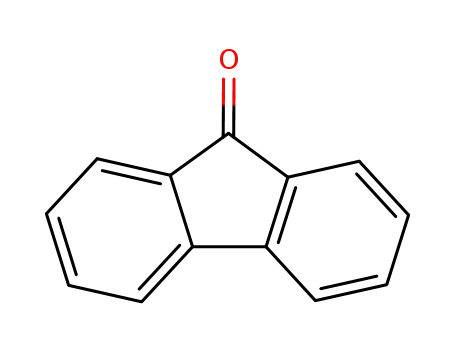
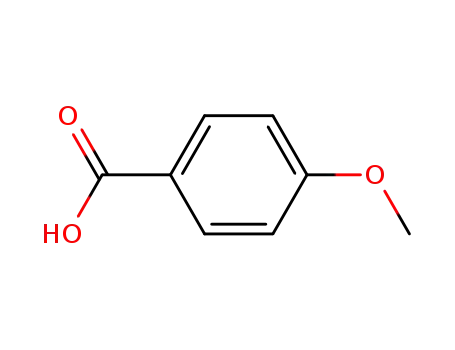
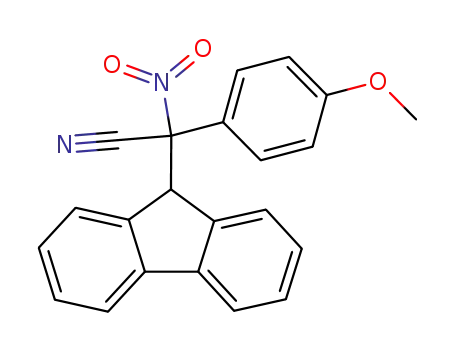
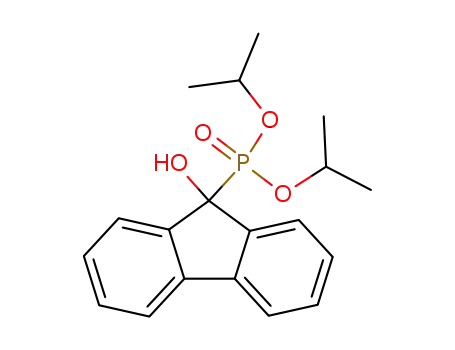
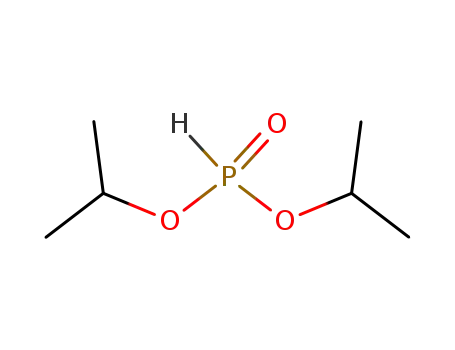
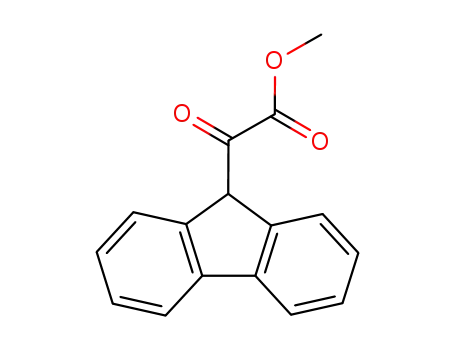
17H-Tetrabenzo[a,c,g,i]fluoren-17-one po...
A novel method for monitoring the transesterification reaction of oil in biodiesel production by estimation of glycerol
Reddy, Sabbasani Rajasekhara,Titu, Devamani,Chadha, Anju
, p. 747 - 754 (2010)
A quantitative method is reported for th...
-
Schiessler,Eldred
, p. 3958 (1948)
-
-
Schoenberg,Awad
, p. 788 (1950)
-
Chromium-assisted oxidations with sodium perborate by phase transfer catalysis
Muzart,N'Ait Ajjou
, p. 575 - 580 (1991)
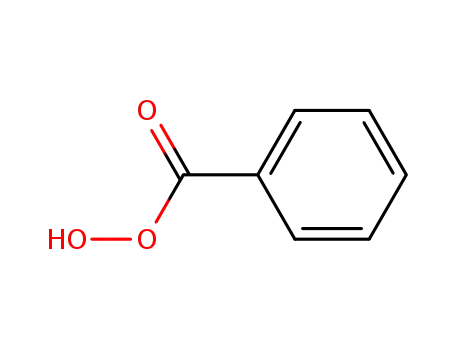
Oxidation by sodium perborate of selecte...
Synthesis of fluorovinyl pyrazolyl (thio)ethers by the reaction of gem-difluoroalkenes with pyrazolin-5-ones (thiones)
Huang, Tao,Zhao, Xianghu,Ji, Xinfei,Wu, Wei,Cao, Song
, p. 61 - 68 (2016)
A mild and efficient method for the prep...
Retro Abramov vs. Rearrangement path competition in hydroxyphosphonate decomposition
Gancarz, Roman,Gancarz, Irena,Deron, Agnieszka
, p. 61 - 69 (2000)
1-hydoxyphosphonates in the presence of ...
Ruthenium-catalyzed cytochrome P-450 type oxidation of alkanes with alkyl hydroperoxides
Murahashi,Oda,Naota,Kuwabara
, p. 1299 - 1302 (1993)
The ruthenium-catalyzed oxidation of alk...
-
Wade et al.
, p. 3724 (1979)
-
Tetracyclic arenes by benzannulation of tricyclic carbene complexes of chromium with alkynes: Chemo-, regio-, and stereoselectivity
Pfeiffer, Juergen,Nieger, Martin,Doetz, Karl Heinz
, p. 1843 - 1857 (1998)
The tricarbonyl chromium complexes 7-9 a...
-
Huntress,Hershberg,Cliff
, p. 2720,2724 (1931)
-
Substituent and pH Effects on the Hydrolysis Modes of 9-(dinitromethyl)-9-alkoxylfluorenes
Hoz, Shmaryahu,Perach, Sara Sima
, p. 4056 - 4059 (1982)
The hydrolysis of 9-(dinitromethyl)-9-al...
On the mechanism of the directed ortho and remote metalation reactions of N,N-dialkylbiphenyl 2-carboxamides
Tilly, David,Fu, Jian-Min,Zhao, Bao-Ping,Alessi, Manlio,Castanet, Anne-Sophie,Snieckus, Victor,Mortier, Jacques
, p. 68 - 71 (2010)
"Chemical Equation Presented" A study co...
C-OH bond cleavage initiated by electron transfer: Electroreduction of 9-fluorenol
Mendkovich, Andrey S.,Syroeshkin, Mikhail A.,Nasybullina, Darya V.,Mikhailov, Mikhail N.,Gultyai, Vadim P.,Elinson, Mikhail N.,Rusakov, Alexander I.
, p. 962 - 973 (2016)
Cyclic voltammetry, chronoamperometry, c...
Novel photo-induced coupling reaction of 9-fluorenylidenemalononitrile with 10-methyl-9,10-dihydroacridine
Jiang, Hong,Liu, You-Cheng,Li, Jing,Wang, Guan-Wu,Wu, Yun-Dong,Wang, Quan-Ming,Mak, Thomas C. W.
, p. 882 - 883 (2002)
9-Fluorenylidenemalononitrile reacts wit...
Selective Oxidation of Arenes in Dry Media under Focused Microwaves
Oussaid, Abdelouahad,Loupy, Andre
, p. 342 - 343 (1997)
Arenes are oxidized into ketones within ...
Different Z/E-selectivity depending upon the length of the acyl side chain in the formation of 2,2′-diacyl-9,9′-bifluorenylidene
Oota, Atsushi,Imai, Toshinobu,Yamazaki, Ayumi,Oba, Toru,Karikomi, Michinori,Minabe, Masahiro
, p. 333 - 335 (2006)
We studied the formation of 2,2′-diacyl-...
Oxidative carbonylation of aromatic hydrocarbons in the system containing Pd or Rh compound, trifluoroacetic acid and its anhydride, and MnO2 or Mn2O3
Kalinovskii,Pogorelov,Gelbshtein,Akhmetov
, p. 1457 - 1462 (2001)
Manganese(II) and manganese(IV) oxides a...
Evidence for rhenaphenanthrene formation and its conversion to fluorenone
Mike, Carl A.,Ferede, Roman,Allison, Neil T.
, p. 1457 - 1459 (1988)
Introduction of 2,2′-dilithiobiphenyl to...
Surface-inspired molecular vanadium oxide catalysts for the oxidative dehydrogenation of alcohols: Evidence for metal cooperation and peroxide intermediates
Werncke, C. Gunnar,Limberg, Christian,Knispel, Christina,Mebs, Stefan
, p. 12129 - 12135 (2011)
On the basis that thiacalix[4]arene (H4T...
CeO2–δ-Modified CuFe2O4 with Enhanced Oxygen Transfer as Efficient Catalysts for Selective Oxidation of Fluorene under Mild Conditions
Huang, Xiubing,Wang, Peng,Zhang, Hean,Guo, Zhengwei,Liu, Jijia,Lu, Guilong,Pang, Guangsheng,Wang, Ge
, p. 91 - 97 (2019)
The design of efficient catalysts for th...
Substitution of 9-(α-bromo-α-arylmethylene)fluorenes by thiolate ions in aqueous acetonitrile
Rappoport, Zvi,Shainyan, Bagrat A.
, p. 871 - 878 (1997)
The substitution of 9-(α-bromo-α-arylmet...
Vitamin B12 supported on graphene oxide: As a bio-based catalyst for selective aerobic oxidation of alcohols
Shaabani, Ahmad,Rashidi Vahid, Adina,Shaabani, Shabnam,Mohammadian, Reza,Nazeri, Mohammad Taghi,Keramati Nejad, Mina
, (2018)
The environmental impact of chemical pro...
Indolopyridines with a bridging heteroatom. 9. Synthesis, structure, and thermolysis of 5-hydroxy-5-(2-pyridyl)-fluorene and -4-azafluorene
Soldatenkov,Kolyadina,Kuleshova,Khrustalev
, p. 817 - 821 (1996)
Treatment of fluorenone or 4-azafluoren-...
Carbene-to-carbene oxygen atom transfer
Kovacs, Dalila,Lee, Ming-Shi,Olson, David,Jackson, James E.
, p. 8144 - 8145 (1996)
-
Pentamethylcyclopentadienyl Half-Sandwich Diazoalkane Complexes of Ruthenium: Preparation and Reactivity
Albertin, Gabriele,Antoniutti, Stefano,Bortoluzzi, Marco,Botter, Alessandra,Castro, Jesús
, p. 5592 - 5602 (2016)
The diazoalkane complexes [Ru(η5-C5Me5)(...
Thermal and photochemical 1,3-dipolar cycloaddition of a sulfine (Fluorenethione S-oxide) to the strained triple bond of cyclooctyne
Adam, Waldemar,Froehling, Bettina,Weinkoetz, Stephan
, p. 9154 - 9155 (1998)
-
Direct proof for a lower reactivity of monomeric vs. dimeric oxidovanadium complexes in alcohol oxidation
Werncke, C. Gunnar,Limberg, Christian,Metzinger, Ramona
, p. 2426 - 2432 (2013)
Previous attempts to synthesize and isol...
Structure of 9-(4-pyridylmethylene)fluorene and 9-(E)-benzylidene-1-azafluorene. Oxidation of 9-(4-pyridylmethylene)fluorene and its condensation with acetylenedicarboxylic acid diester
Kolyadina,Soldatenkov,Gridunova,Prostakov
, p. 833 - 836 (1998)
It war found by x-ray diffraction analys...
Dispiro[fluorene-9,5′-[1,2,3,4]tetrathiane-6′, 9″-fluorene]
Linden,Gebert,Heimgartner
, p. 764 - 766 (2001)
The tetrathiane ring of the title compou...
Synthesis of Substituted Quinazolin-4(3H)-imines from Aryldiazonium Salts, Nitriles and 2-Cyanoanilines via A Metal-Free Tandem Approach
Ramanathan, Mani,Liu, Yi-Hung,Peng, Shie-Ming,Liu, Shiuh-Tzung
, p. 5840 - 5843 (2017)
A transition metal-free synthesis of mul...
Fluorenone Monomer Dianion Studied by 1H and 13C NMR and Charge Density Distribution
Hirayama, Masatoshi,Ohhata, Hiroshi
, p. 2751 - 2756 (1987)
The diamagnetic monomer dianion of fluor...
Selective Aerobic Oxidation of Csp3-H Bonds Catalyzed by Yeast-Derived Nitrogen, Phosphorus, and Oxygen Codoped Carbon Materials
Ju, Zhao-Yang,Song, Li-Na,Chong, Ming-Ben,Cheng, Dang-Guo,Hou, Yang,Zhang, Xi-Ming,Zhang, Qing-Hua,Ren, Lan-Hui
supporting information, p. 3978 - 3988 (2022/03/16)
Nitrogen, phosphorus, and oxygen codoped...
Nanostructured Manganese Oxides within a Ring-Shaped Polyoxometalate Exhibiting Unusual Oxidation Catalysis
Sato, Kai,Yonesato, Kentaro,Yatabe, Takafumi,Yamaguchi, Kazuya,Suzuki, Kosuke
supporting information, (2021/12/30)
Nanosized manganese oxides have recently...
[Fe(bpy)3]2+-based porous organic polymers with boosted photocatalytic activity for recyclable organic transformations
Liu, Hong-Kun,Lei, Yi-Fei,Tian, Peng-Ju,Wang, Hui,Zhao, Xin,Li, Zhan-Ting,Zhang, Dan-Wei
supporting information, p. 6361 - 6367 (2021/03/22)
Three rigid metal porous organic polymer...
Nitrosoarene-Catalyzed HFIP-Assisted Transformation of Arylmethyl Halides to Aromatic Carbonyls under Aerobic Conditions
Pradhan, Suman,Sharma, Vishali,Chatterjee, Indranil
supporting information, p. 6148 - 6152 (2021/08/03)
A rare metal-free nucleophilic nitrosoar...