
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Preparation |
Cyanoacetamide can be prepared from ethyl cyanoacetate and ammonia. Cool the ethyl cyanoacetate to below 200°C, add concentrated ammonia water to react, and the mixture turns from turbid to clear. After cooling in cold water for 20 minutes, a precipitate is precipitated. After filtration and drying, the crude product is obtained, and then the crude product of cyanoacetamide is added. In the boiling ethanol, after dissolving it, add a small amount of activated carbon for decolorization and purification, filter, cool the filtrate to separate out the precipitate, and dry it at 80-100 ℃ to obtain the fine cyanoacetamide. |
|
Purification Methods |
Crystallise the amide from MeOH/dioxane (6:4), then water and dry it over P2O5 under vacuum. [Beilstein 2 IV 1891.] |
|
Definition |
2-Cyanoacetamide is an acetic amide with a nitrile functional group. It is a codon of glutamine that directs the placement of glutamine into a polypeptide. |
|
General Description |
Cyanoacetamide is the starting reagent for vitamin B6 synthesis. It reacts with reducing carbohydrates in borate buffer to give intense fluorescence andis useful for the automated analysis of carbohydrates as borate complexes. |
InChI:InChI:1S/C3H4N2O/c4-2-1-3(5)6/h1H2,(H2,5,6)
Nowadays, most peptides are chemically a...
Different series of novel pyrazole and p...
Many natural or synthetic chalcones have...
A preliminary simulation of bioactive co...
The regioselective hydration of dinitril...
Treatment of an α-amino acid with N-brom...
Abstract: A new series of fused tricycli...
The invention relates to a synthesis met...
The embodiment of the invention provides...
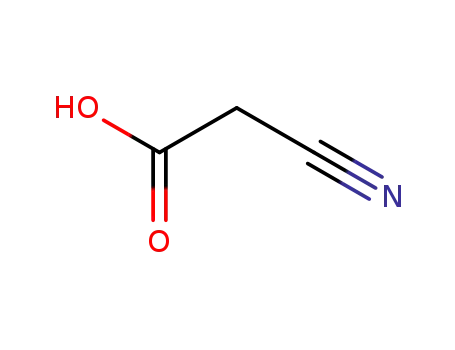
cyanoacetic acid

phenol

phenyl 2-cyanoacetate

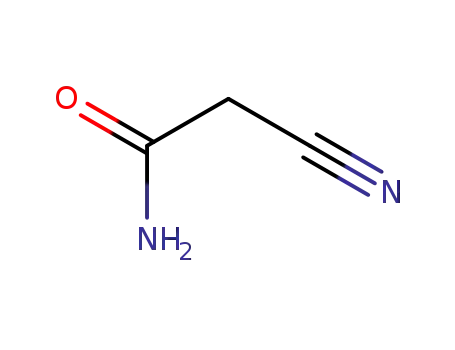
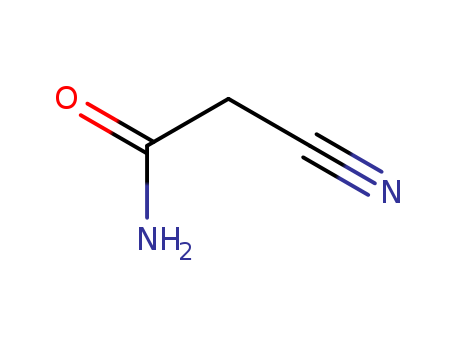
cyanoacetic acid amide
| Conditions | Yield |
|---|---|
|
at 160 ℃;
|
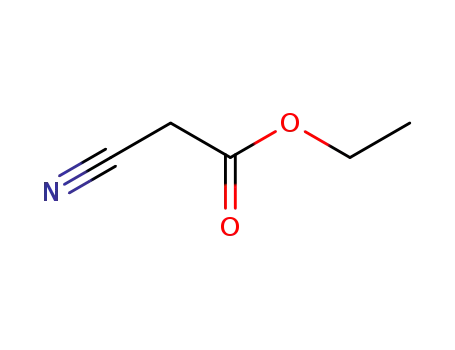
ethyl 2-cyanoacetate


cyanoacetic acid amide
| Conditions | Yield |
|---|---|
|
With
ammonia;
|
100% |
|
With
ammonia;
In
tetrahydrofuran; water;
at 20 ℃;
for 2h;
|
93% |
|
With
ammonium hydroxide;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
|
91% |
|
With
ammonia;
In
water;
for 2h;
|
70% |
|
With
ammonium hydroxide;
Heating;
|
70% |
|
With
ammonia;
In
water;
for 2h;
Cooling with ice;
|
60% |
|
With
ammonia;
In
water;
for 1h;
|
38% |
|
With
ethanol; ammonia;
|
|
|
With
ammonia; ammonium chloride;
at 0 ℃;
|
|
|
With
ammonia; water;
|
|
|
With
ammonium hydroxide; water;
at -15 ℃;
|
|
|
With
ammonia;
Darstellung;
|
|
|
With
ammonia;
at 20 ℃;
|
|
|
With
ammonia;
at 20 ℃;
for 1h;
|
|
|
With
ammonia;
at 20 - 70 ℃;
|
|
|
With
ammonia;
In
ethanol;
Reflux;
|
|
|
With
ammonia;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
|
|
With
ammonia;
In
N,N-dimethyl-formamide;
for 8h;
Reflux;
|
|
|
With
ammonium hydroxide;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
|
|
|
With
ammonia;
In
ethanol;
for 4h;
Reflux;
|
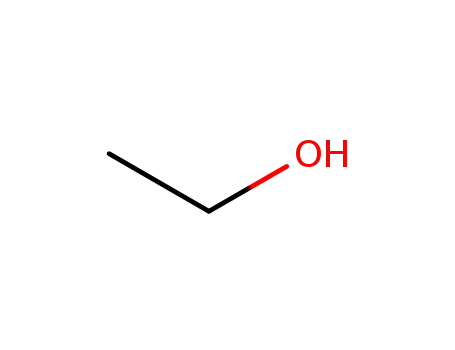
ethanol
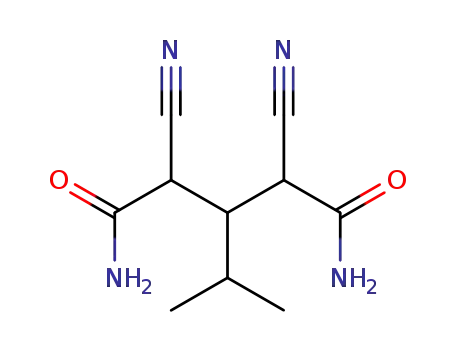
2,4-dicyano-3-isopropyl-glutaric acid diamide
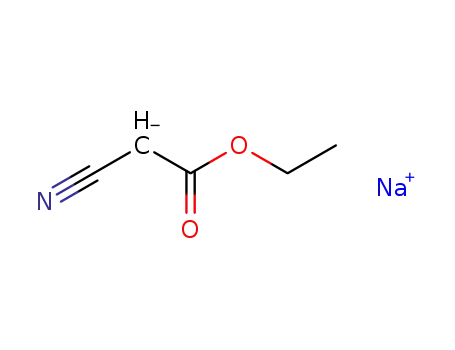
sodium cyanoacetic acid ethyl ester

cyanoacetic acid
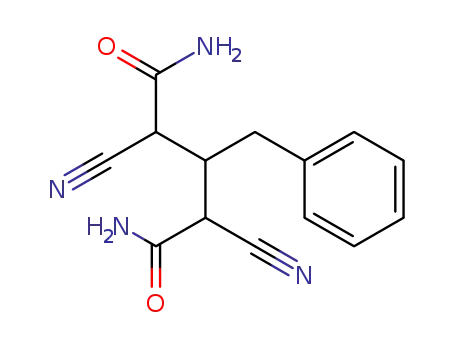
3-benzyl-2,4-dicyano-glutaric acid diamide
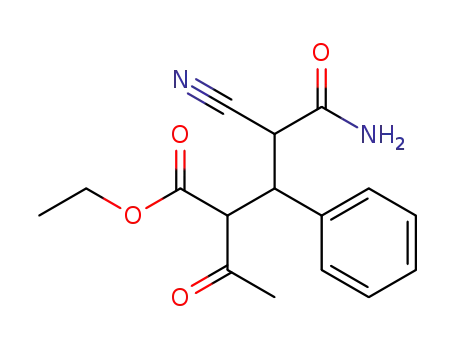
2-acetyl-4-cyano-3-phenyl-glutaramic acid ethyl ester
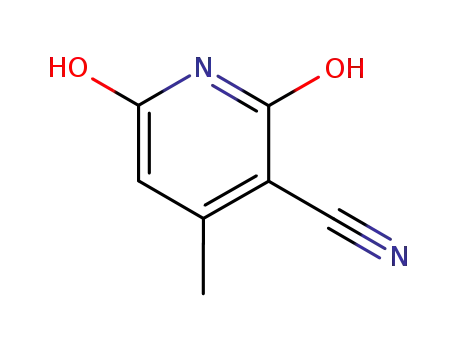
2,6-dihydroxy-3-cyano-4-methylpyrimidine
3-(R,S),5-(R,S)-3,5-dicyano-4,4-dimethyl-2,6-dioxopiperidine
