
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
General Description |
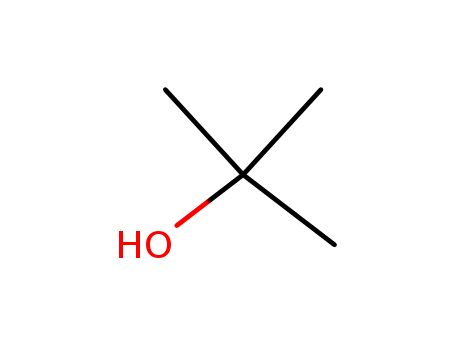
Tert-butyl alcohol, also known as 2-methylpropan-2-ol, is a colorless organic compound with a strong, sweet odor. It is commonly used as a solvent and as a raw material in the production of other chemicals, such as tert-butyl acetate and tert-butyl chloride. Tert-butyl alcohol is flammable and can be harmful if inhaled, ingested, or absorbed through the skin. It is also known to cause irritation to the eyes, skin, and respiratory system. Tert-butyl alcohol is considered to have low acute toxicity, but prolonged exposure can lead to adverse health effects. As a result, proper handling and safety precautions should be taken when working with this chemical. |
InChI:InChI=1/C4H10O/c1-4(2,3)5/h5H,1-3H3
Direct electron-transfer and electrocata...
The products from the alkylation of silv...
-
-
The kinetics of the oxidation of hydrazi...
The solvolysis rate constants (kobs) of ...
Birnessite-type manganese oxides (M-OL-1...
Pure liquid water is thought to contain ...
The use of optoacoustic spectroscopy per...
The investigation into the active copper...
Dual-metalloporphyrins catalytic system ...
It was found that the differential react...
Co-metabolic bioremediation is a promisi...
The selective oxidation reactions of iso...
We have constructed the pseudoternary ph...
Precise first-order rate constants for t...
A series of tetrakis(pentafluorophenyl)p...
Solvolytic rate constants for 1-adamanty...
The reaction of 2,4,6-triphenyl- and 2,3...
Compound-specific analysis of stable car...
Voltammetry of cytochrome P450 (cyt P450...
An expanded porphyrin-biscopper hexaphyr...
A sensitive, reliable, and reproducible ...
The direct sources of aliphatic acids in...
Fast addition of highly polar organometa...
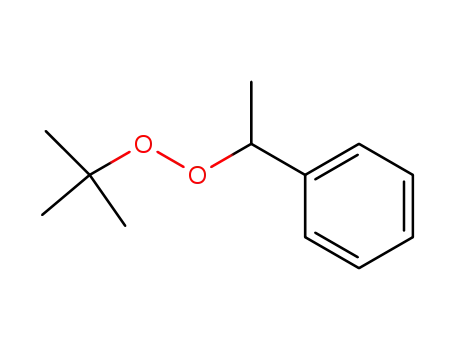
1,1-dimethylethyl-1-phenylethyl peroxide

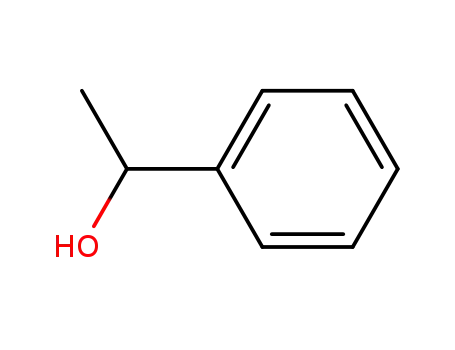
1-Phenylethanol

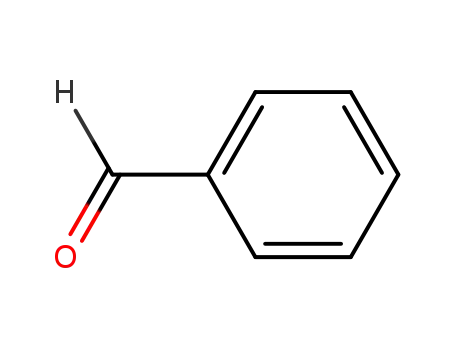
benzaldehyde

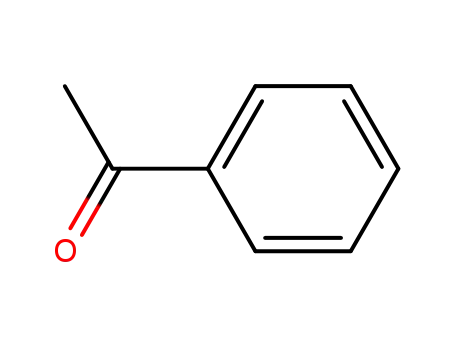
acetophenone

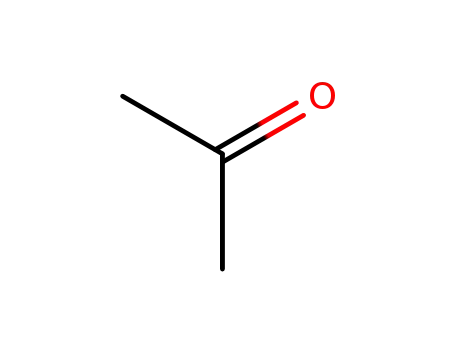
acetone

tert-butyl alcohol
| Conditions | Yield |
|---|---|
|
In
chlorobenzene;
at 129.2 ℃;
Product distribution;
Rate constant;
also with styrene, dimethylaniline, and 2,6-di-tert-butyl-p-cresol (radical traps);
|
78.8% 3.12% 1.1% 26.2% 49.4% |
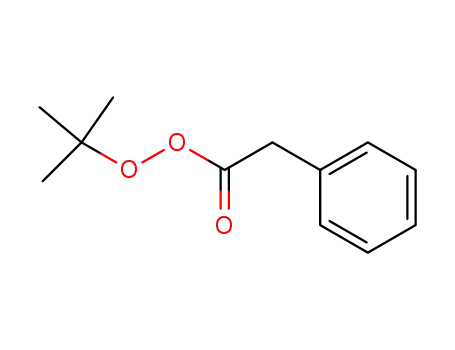
t-butyl phenylperacetate

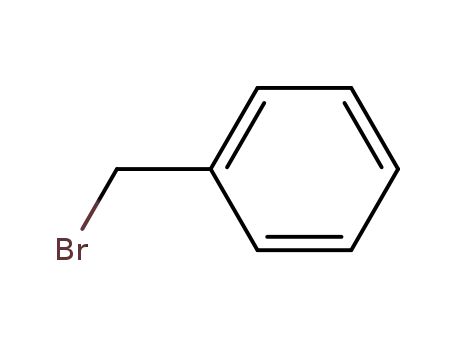
benzyl bromide


acetone

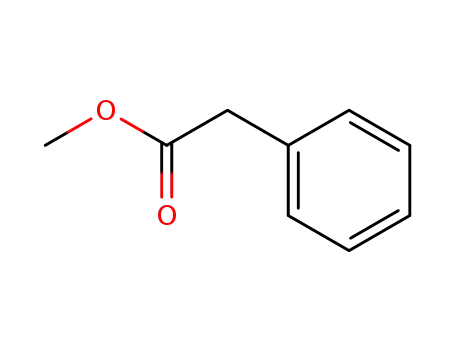
benzeneacetic acid methyl ester

tert-butyl alcohol
| Conditions | Yield |
|---|---|
|
With
toluene-p-sulfonyl bromide;
In
benzene;
at 70 ℃;
for 40h;
Further byproducts given;
|
139.7 mg 51.6 mg 20.5 mg 4.0 mg |

diazomethane
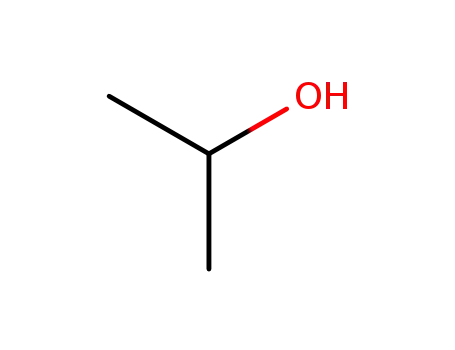
isopropyl alcohol
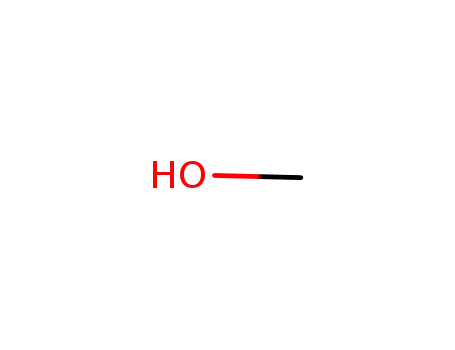
methanol
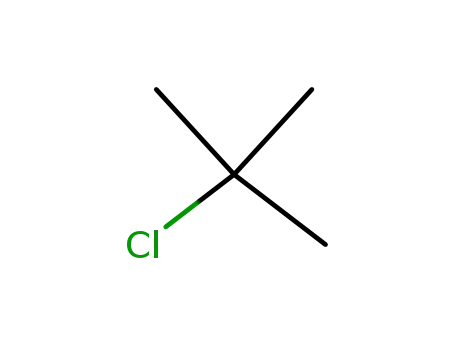
tertiary butyl chloride
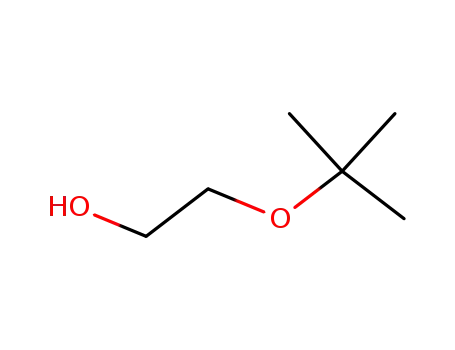
tert-butyl glycidyl ether
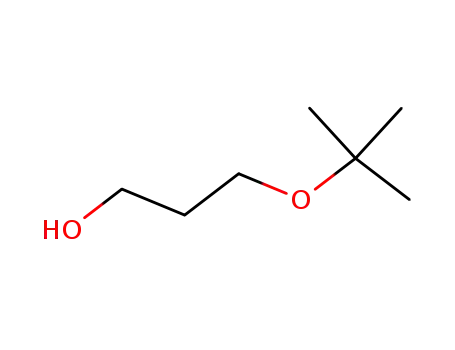
3-(tert-butoxy)propan-1-ol
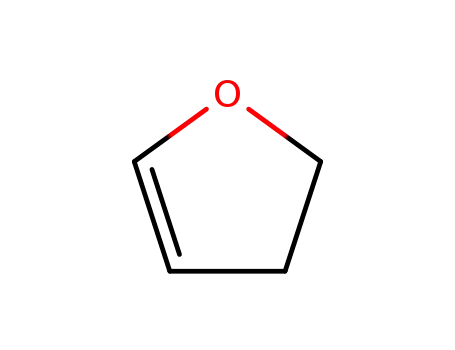
2,3-dihydro-2H-furan
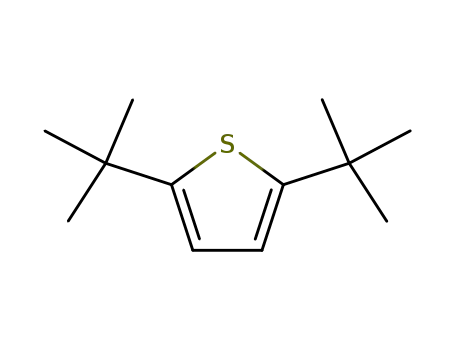
2,5-di-tert-butylthiophene
