
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Clinical application |
Hexafluoroisopropanol(1,1,1,3,3,3-Hexafluoro-2-propanol) can be used to prepare a variety of high-end chemicals such as fluorosurfactants, fluorine-containing emulsifiers, fluorine-containing pharmaceuticals, and used as a solvent or cleaning agent for electronics industry. The drug can be used as a fluorinated solvent to increase the efficiency of the reaction of rhodium (I)-catalyzed [4 + 2] intramolecular addition reaction of ether bound alkynyl diene and the [5 + 2] cycloaddition of alkynyl vinyl cyclopropane. The drug is a liquid peptide chemical solvent, a highly soluble solvent for peptide and peptide intermediates. The drug can be used for the analysis of multiple polymers. |
|
Hazards |
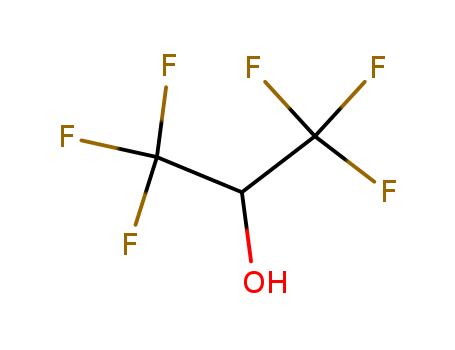
1,1,1,3,3,3-Hexafluoro-2-propanol?derives from a propan-2-ol. It is a clear colorless oily liquid with an aromatic odor. (NTP, 1992) Combustible, may cause burns to skin, eyes and mucous membranes. |
|
Preparation |
1,1,1,3,3,3-Hexafluoro-2-propanol is prepared from hexafluoropropylene through hexafluoroacetone, which is then hydrogenated. (CF3)2CO + H2 → (CF3)2CHOH |
|
Definition |
ChEBI: An organofluorine compound formed by substitution of all the methyl protons in propan-2-ol by fluorine. It is a metabolite of inhalation anesthetic sevoflurane. |
|
General Description |
1,1,1,3,3,3-Hexafluoro-2-propanol is a solution-phase peptide chemistry solvent. This fluorinated polar solvent of high ionizing power facilitates Friedel–Crafts-type reactions, using covalent reagents in the absence of a Lewis acid catalyst. It also enhances the efficiency of rhodium(I)-catalyzed [4+2] intramolecular cycloaddition of ether-tethered alkynyl dienes and [5+2] cycloaddition of alkynyl vinylcyclopropanes. 1,1,1,3,3,3-Hexafluoro-2-propanol clusters catalyzes the epoxidation of cyclooctene and 1-octene with hydrogen peroxide. |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
1,1,1,3,3,3-Hexafluoro-2-propanol is incompatible with acids, acid chlorides and oxidizing agents. |
|
Fire Hazard |
1,1,1,3,3,3-Hexafluoro-2-propanol is probably combustible. |
|
Potential Exposure |
A specialty solvent for some poly mers; a lavatory reagent. |
|
Shipping |
UN1760 Corrosive liquids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material. |
|
Purification Methods |
Distil it from 3A molecular sieves, retaining the middle fraction. It has been prepared by reduction of hexafluoroacetone in tetrahydrofuran (THF), In this case hexafluoropropanol forms a stable 1:1 complex which distils at 99-100o/760mm (n 25 1.3283), The complex is decomposed by mixing with 20% oleum and distilling in a vacuum, and the distillate is redistilled to give pure hexafluoropropan-2-ol with b 59o/760mm. The 1H NMR shows a doublet at 4.52ppm (JH,H 2Hz). The benzoyl derivative, [10315-85-2] M 272.1, has m 53.9o after crystalllisation from pentane at -50o, and its IR has at 1760cm-1. [Middleton & Lindsey J Am Chem Soc 86 4948 1964, Urry et al. J Org Chem 32 347 1967.] It has very high peptide solubilising properties, alone or with CH2Cl2 [use as a solvent: Narita et al. Bull Chem Soc Jpn 61 281 1988, Biochemistry 29 2639 1990.] It is CORROSIVE, causes severe eye irritation. |
|
Incompatibilities |
HFIP is incompatible with acids, acid chlorides, and oxidizing agents. |
|
Waste Disposal |
May be incinerated. In accor dance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be dis posed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste contain ing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. |
InChI:InChI=1/C11H3F5N2O2/c12-5-6(13)8(15)10(9(16)7(5)14)20-11(19)4-1-17-3-18-2-4/h1-3H
Axially chiral biaryls are ubiquitous st...
The invention discloses a preparation me...
The invention provides a method for synt...
A novel nickel N-heterocyclic carbene ca...
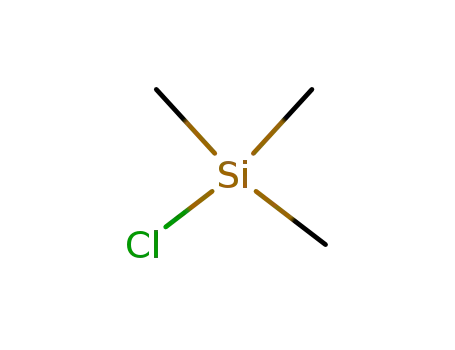
chloro-trimethyl-silane

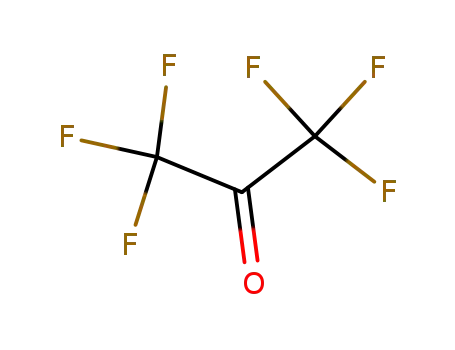
Hexafluoroacetone

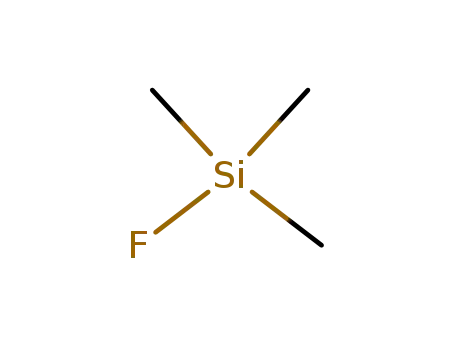
trimethylsilyl fluoride

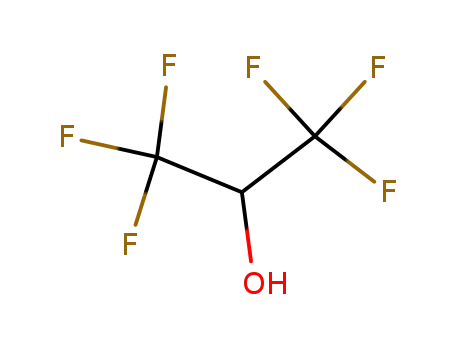
1,1,1,3',3',3'-hexafluoro-propanol

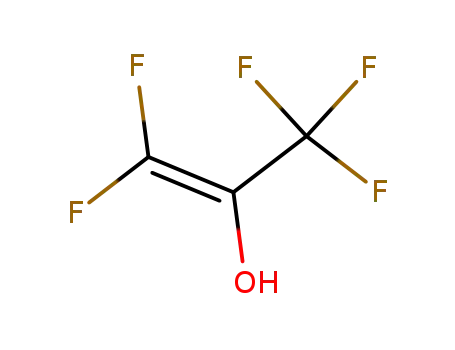
pentafluoroprop-1-en-2-ol
| Conditions | Yield |
|---|---|
|
chloro-trimethyl-silane; Hexafluoroacetone;
With
magnesium;
In
N,N-dimethyl-formamide;
at -20 - 0 ℃;
for 1h;
With
sulfuric acid;
at -30 - 0 ℃;
for 4h;
Further stages. Title compound not separated from byproducts.;
|
7.1 g |

Hexafluoroacetone

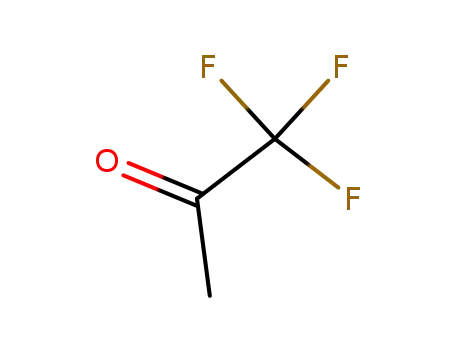
1,1,1-trifluoro-2-propanone


1,1,1,3',3',3'-hexafluoro-propanol

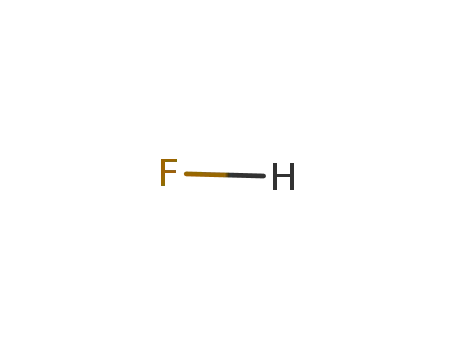
hydrogen fluoride
| Conditions | Yield |
|---|---|
|
With
hydrogen;
catalyst;
|
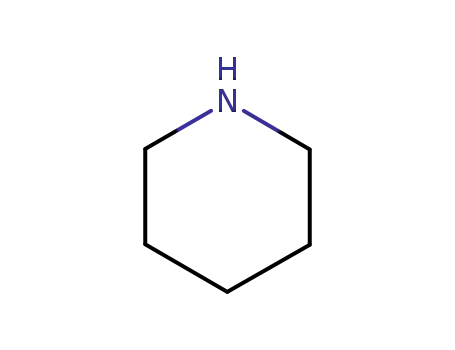
piperidine

Hexafluoroacetone
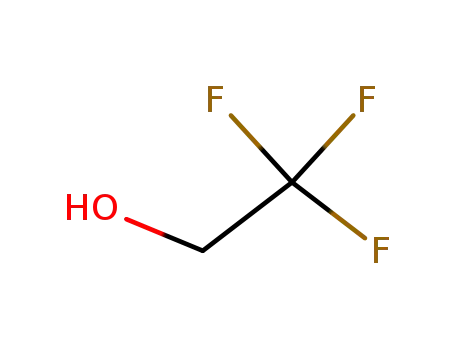
2,2,2-trifluoroethanol
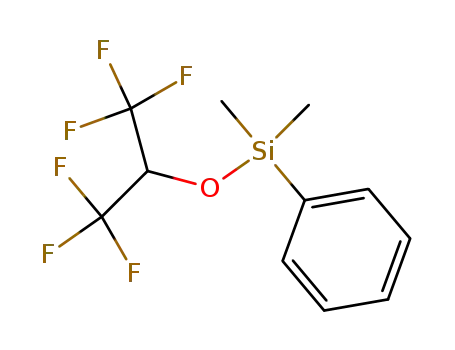
Dimethyl-phenyl-(2,2,2-trifluoro-1-trifluoromethyl-ethoxy)-silane
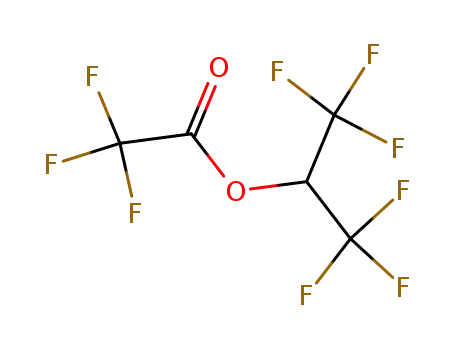
hexafluoroisopropyl atrifluoroacetate
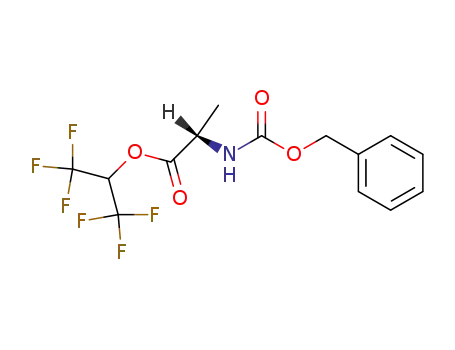
Z-Ala-O-hexafluorisopropyl
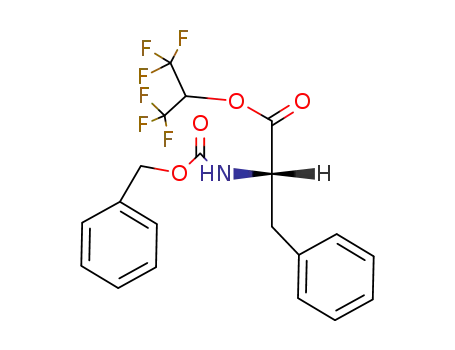
Z-L-Phe-OCH(CF3)2
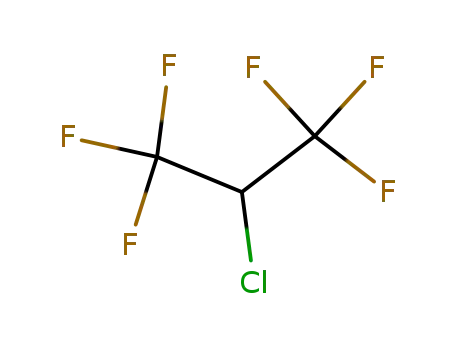
2-chloro-1,1,1,3,3,3-hexafluoropropane
