
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Preparation |
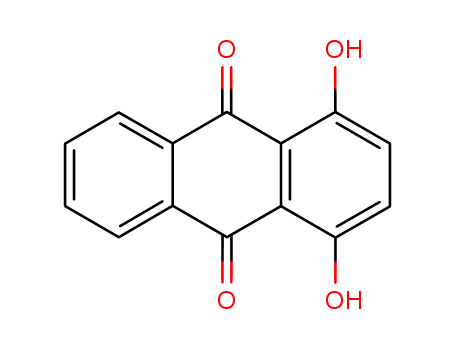
commonly known as Quinizarin. (a) Boric acid, boric acid and mercury, boric acid and nitrous acid or nitrous acid and mercury in the presence of Anthracene-9,10-dione with sulfuric acid; (b) In the presence of boric acid and nitrous acid, treated with sulfuric acid ?1-Hydroxyanthracene-9,10-dione or 2-Hydroxyanthracene-9,10-dione, (c)In the presence of boric acid, treated with sulfuric acid 1-Hydroxy-4-nitroanthracene-9,10-dione?or?1,4-Dichloroanthracene-9,10-dione?; (d) in the Boric acid and where did in the presence of sulfuric acid, Phthalic anhydride and 4-Chlorophenol?or Hydroquinone condensation, closed loop. |
|
Synthesis Reference(s) |
Synthesis, p. 633, 1974 DOI: 10.1055/s-1974-23387Tetrahedron Letters, 28, p. 1533, 1987 DOI: 10.1016/S0040-4039(01)81035-2 |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Poison by intravenous route. Moderately toxic by intraperitoneal route. Mutation data reported. An eye irritant. A weak allergen. When heated todecomposition it emits acrid smoke and irritating fumes. |
|
Purification Methods |
Crystallise quinizarin from glacial acetic acid. [Beilstein 8 H 450, 8 IV 3260.] |
|
Definition |
ChEBI: A dihydroxyanthraquinone having the two hydroxy substituents at the 1- and 4-positions; formally derived from anthraquinone by replacement of two hydrogen atoms by hydroxy groups |
|
General Description |
1,4-Dihydroxyanthraquinone is an organic dye molecule with an aromatic structure. It is a derivative of anthraquinone bearing hydroxyl moieties. They may find uses in pharmacological, biochemical and dye industries. Anthraquinone dye are resistant to degradation. |
|
Properties and Applications |
orange. Orange red powder. Insoluble in water, soluble in ether, in strong base in certain solubility, but soluble in organic solvent oil, etc. In concentrated sulfuric acid in green yellow fluorescence. Mainly used for oil coloring. Also used in all kinds of plastic and resin, light industry products coloring. |
InChI:InChI=1/C14H8O4/c15-9-5-6-10(16)12-11(9)13(17)7-3-1-2-4-8(7)14(12)18/h1-6,15-16H
The invention relates to the technical f...
The invention discloses a synthesis meth...
Background: A phytochemical study on med...
The invention discloses a fluorescent mo...
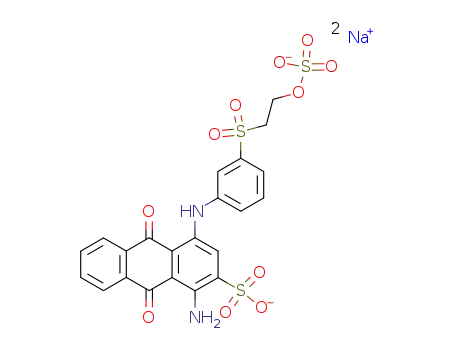
reactive blue 19

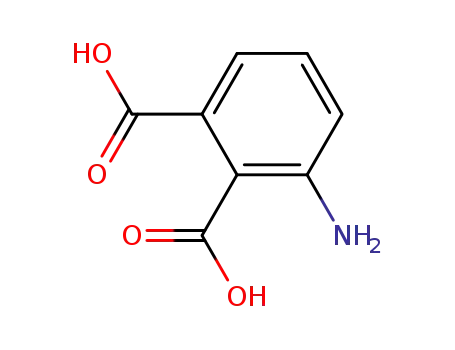
3-aminophthalic acid

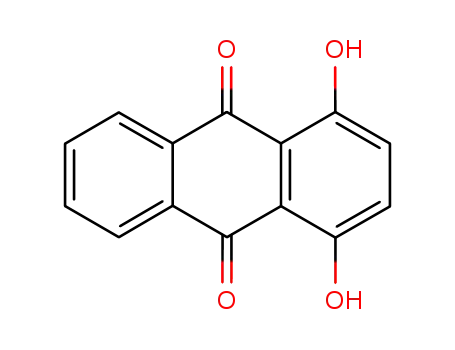
1,4-dihydroxy-9,10-anthracenedione

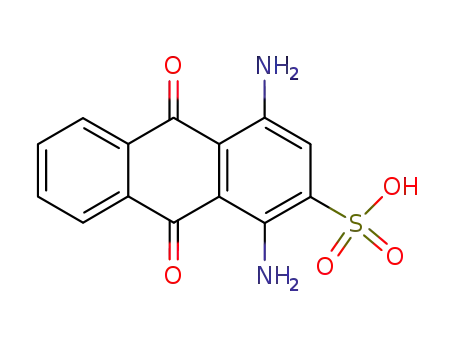
1,4-diamino-9,10-dioxo-9,10-dihydro-anthracene-2-sulfonic acid

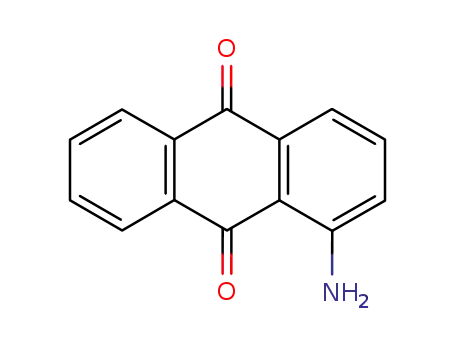
1-amino-9,10-anthracenedione

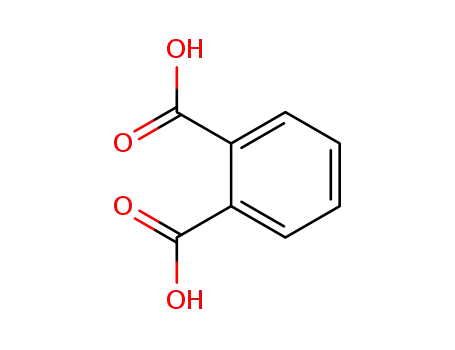
benzene-1,2-dicarboxylic acid
| Conditions | Yield |
|---|---|
|
With chitosan/polyaniline/CdS nanocomposite; for 2h; pH=6; pH-value; Catalytic behavior; Kinetics; Irradiation;
|
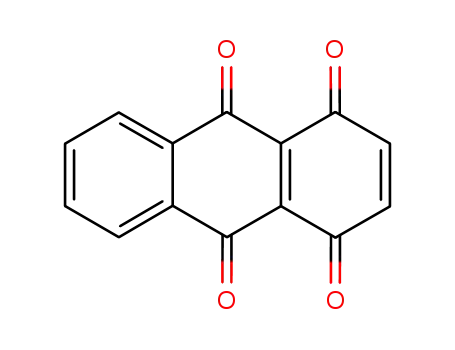
anthracene-1,4,9,10-tetraone


1,4-dihydroxy-9,10-anthracenedione


benzene-1,2-dicarboxylic acid
| Conditions | Yield |
|---|---|
|
With water; Product distribution; Thermodynamic data; Mechanism; ΔrH0m, ΔrG0m;
|
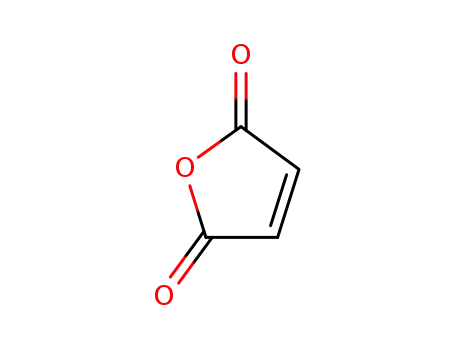
maleic anhydride
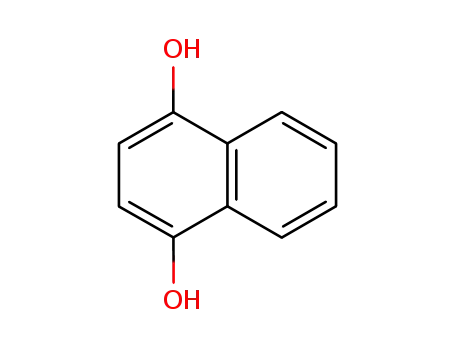
1,4-Dihydroxynaphthalene
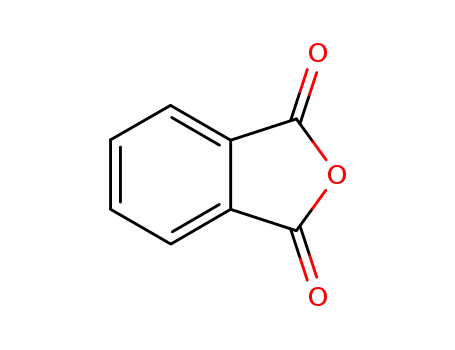
phthalic anhydride
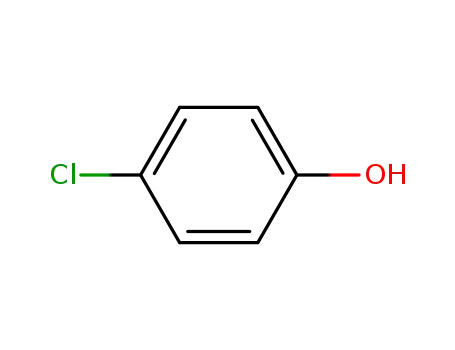
4-chloro-phenol
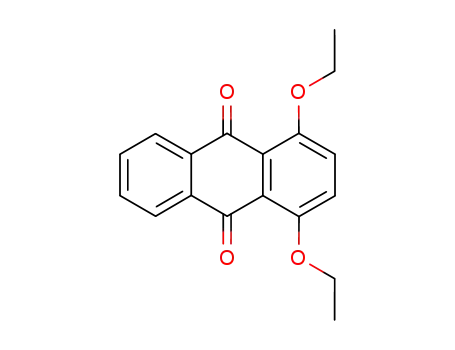
1,4-Diethoxy-9,10-anthraquinone
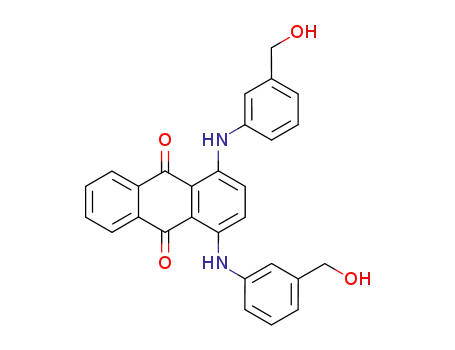
1,4-bis-(3-hydroxymethyl-anilino)-anthraquinone
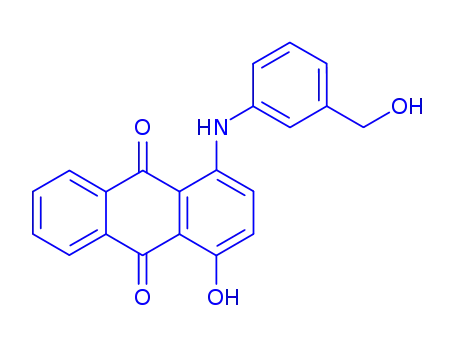
1-hydroxy-4-(3-hydroxymethyl-anilino)-anthraquinone
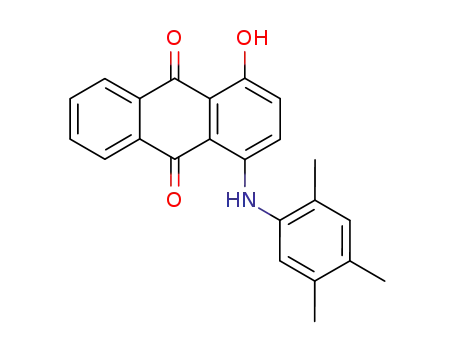
1-hydroxy-4-(2,4,5-trimethyl-anilino)-anthraquinone
