
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Flammability and Explosibility |
Flammable |
|
Biochem/physiol Actions |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is a water soluble condensing reagent. EDAC is generally utilized as a carboxyl activating agent for amide bonding with primary amines. Additionally, it reacts with phosphate groups. It has been utilized in peptide synthesis, crosslinking proteins to nucleic acids as well as preparation of immunoconjugates. |
|
Synthesis |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride can be used for the synthesis of amides.?It is used as a coupling agent in the synthesis?of esters from carboxylic acids using dimethylaminopyridine as the catalyst. |
|
Purification Methods |
It is an excellent H2O-soluble peptide coupling reagent. It is purified by dissolving (ca 1g) in CH2Cl2 (10mL) at room temperature and then add dry Et2O (~110mL) dropwise and the crystals that separate are collected, washed with dry Et2O, recrystallised from CH2Cl2/Et2O and dried in a vacuum over P2O5. It is important to work in a dry atmosphere or work rapidly and then dry the solid as soon as possible. The material is moderately hygroscopic, but once it becomes wet it reacts slowly with H2O. Store it away from moisture at -20o to slow down the hydrolysis process. The free base has b 47-48o/0.27mm, 53-54o/0.6mm, n 1.4582. The methiodide is recrystallised from CHCl3/EtOAc, the crystals are filtered off, washed with dry Et2O, recrystallised from CHCl3/Et2O, and dried in vacuo over P2O5, m 93-95o, 94-95o. [Sheehan et al. J Am Chem Soc 87 2492 1965, Sheehan & Cruickshank Org Synth Coll Vol V 555 1973.] § A polymer bound version is commercially available. |
|
General Description |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC or EDCI·HCl) is a water-soluble carbodiimide commonly used as a coupling agent in peptide synthesis and other chemical reactions, particularly for activating carboxylic acids to form amide or ester bonds. It is frequently employed alongside additives like HOBt or NHS to enhance efficiency and minimize side reactions. EDC has been utilized in diverse applications, including the synthesis of opioid receptor ligands, α-keto esters/amides, and peptide moieties, as well as in surface immobilization strategies for biosensors or biocompatible coatings. Its mild reaction conditions and versatility make it a widely adopted reagent in organic and bioconjugate chemistry. **Returned paragraph:** 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC or EDCI·HCl) is a versatile coupling agent widely used in organic and bioconjugate chemistry for activating carboxylic acids to form amide or ester bonds. It is particularly valuable in peptide synthesis, biosensor development, and the preparation of bioactive compounds due to its water solubility and compatibility with mild reaction conditions. EDC is often paired with additives like HOBt or NHS to improve reaction efficiency and selectivity. Its applications span pharmaceutical synthesis, surface modification, and the creation of functional biomaterials. *(Note: The conclusion integrates relevant uses from the provided abstracts while omitting literature-specific descriptions.)* |
InChI:InChI=1/C7H17N3.ClH/c1-4-8-9-6-5-7-10(2)3;/h4-7H2,1-3H3;1H/b9-8+;
The invention relates to a 1 - ethyl - (...
The invention belongs to the field of or...
The invention relates to substituted dih...
Acrylamide derivatives represented by Ge...
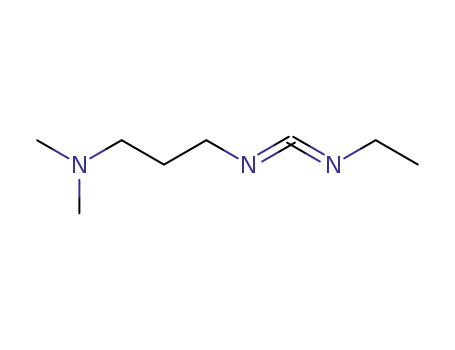
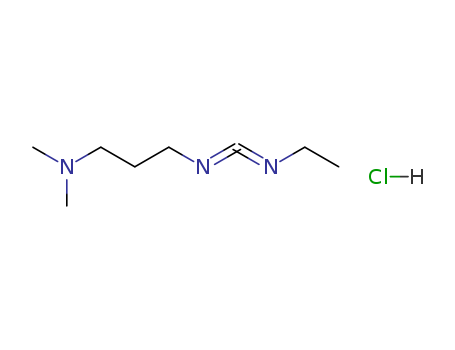
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

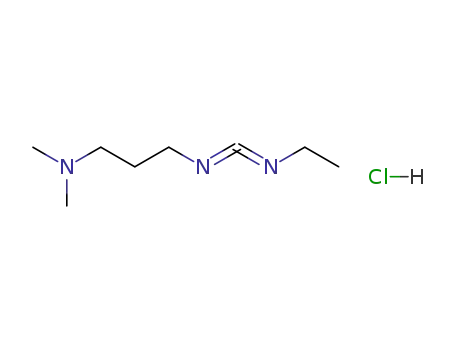
1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride
| Conditions | Yield |
|---|---|
|
With
triethylamine hydrochloride;
In
acetonitrile;
at 15 ℃;
for 1h;
Temperature;
|
92% |
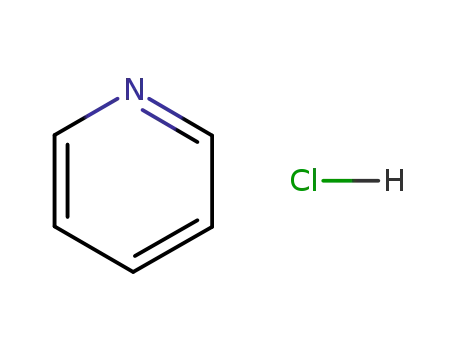
pyridine hydrochloride


N-(3-dimethylaminopropyl)-N-ethylcarbodiimide


1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride
| Conditions | Yield |
|---|---|
|
In
dichloromethane;
at 30 ℃;
Solvent;
Temperature;
|
71.2 g |

pyridine hydrochloride

N-(3-dimethylaminopropyl)-N-ethylcarbodiimide
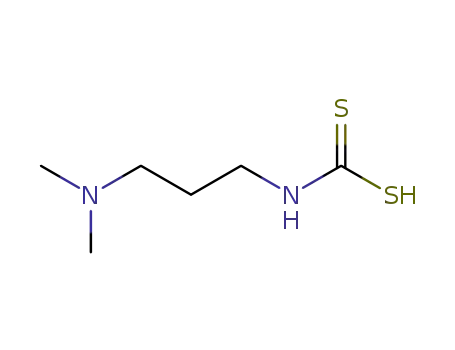
N-(3-Dimethylamino-propyl)-dithiocarbamidsaeure
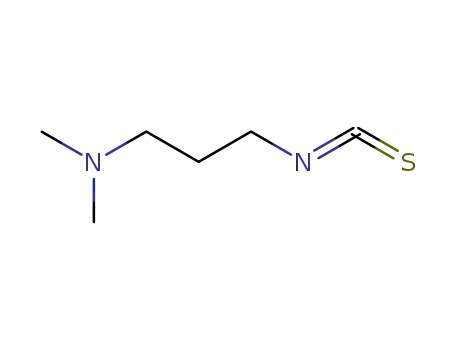
3-(dimethylamino)propyl isothiocyanate
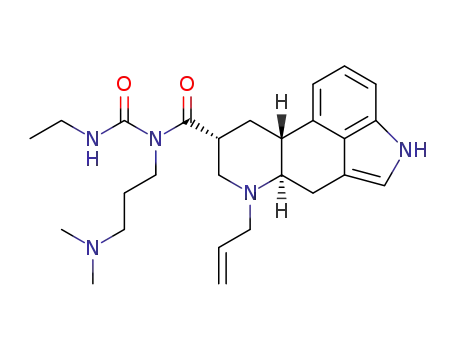
cabergoline
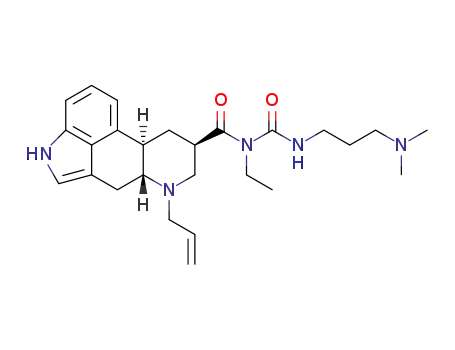
N-<<<3-(dimethylamino)propyl>amino>carbonyl>-N-ethyl-6-(2-propenyl)-ergoline-8β-carboxamide
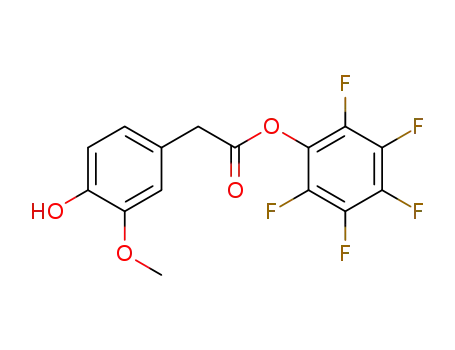
homovanillic acid pentafluorophenyl ester
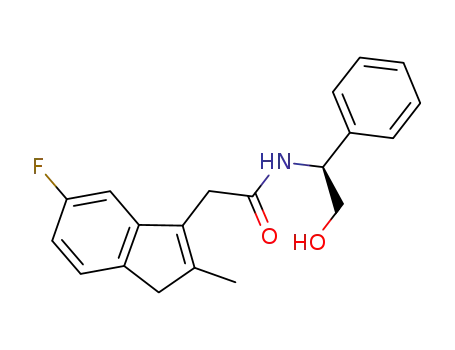
5-fluoro-2-methyl-3-(N-(S-α-hydroxylmethyl)benzyl)-indenylacetamide
