
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Purification Methods |
Crystallise the iodo compound from warm trifluoroacetic acid and dry it over NaOH pellets. Recrystallise it also from Me2CO/pet ether. Its melting point depends on the heating rate. [Spyroudis & Varvoglis Synthesis 445 1975, application: Almond et al. Org Synth 66 132 1988.] |
|
General Description |
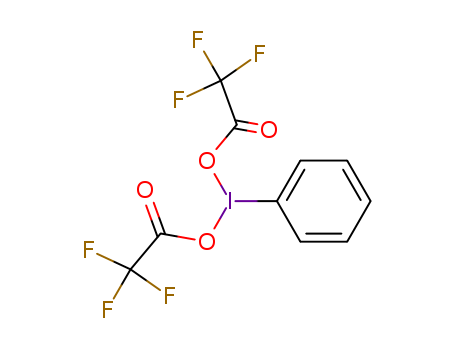
[Bis(trifluoroacetoxy)iodo]benzene (PIFA) is a hypervalent iodine(III) reagent widely used in organic synthesis for oxidative transformations, including intramolecular cyclizations, oxidative couplings, and decarboxylative alkylations. It serves as a versatile oxidant under mild conditions, enabling selective reactions such as the formation of azacarbocyclic spirodienones, oxidative dimerization of thiophenes, and metal-free C–H functionalizations. Its reactivity is often enhanced by additives like BF3·Et2O or photocatalysts, facilitating efficient bond formations without heavy metals. PIFA is particularly valued for its ability to generate radical intermediates and its compatibility with complex substrates, making it useful in pharmaceutical and materials chemistry. |
InChI:InChI=1/C10H3F6IO4/c11-9(12,13)7(18)20-5-3-1-2-4(17)6(5)21-8(19)10(14,15)16/h1-3H
The crystal and molecular structures of ...
The direct synthesis of aryl(2,4,6-trime...
Easy and effective preparations of the n...
The synthesis of salutaridine derivative...
-
-
An easy, safe, and effective method for ...
Hypervalent iodine (HVI) reagents are em...
We found that the oxygen atom of water i...
Organosilanes are synthetically useful r...
α-Arylation of α,β-unsaturated ketones c...
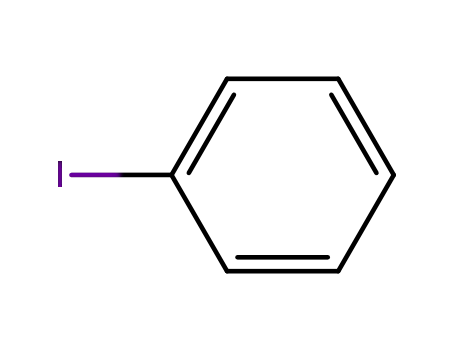
iodobenzene

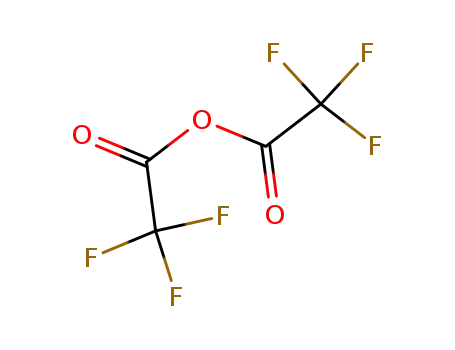
trifluoroacetic anhydride

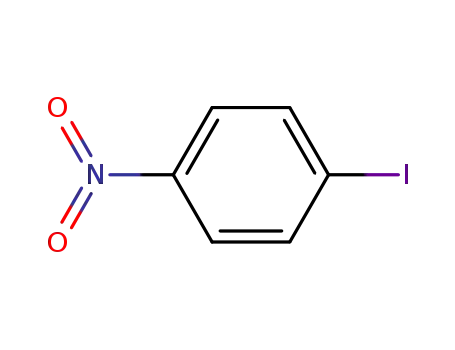
p-nitrobenzene iodide

μ-oxo

![bis-[(trifluoroacetoxy)iodo]benzene](/upload/2025/4/206e18b4-dae3-4d4d-9c84-3f087e5efb89.png)
bis-[(trifluoroacetoxy)iodo]benzene
| Conditions | Yield |
|---|---|
|
With nitric acid; acetic anhydride; In dichloromethane; at -20 ℃; for 0.666667h;
|
2.5% 2 g |
|
With nitric acid; acetic anhydride; In dichloromethane; at -20 ℃; for 0.666667h; Product distribution; variation of temperature, reagent, time;
|
2.5% 2 g |
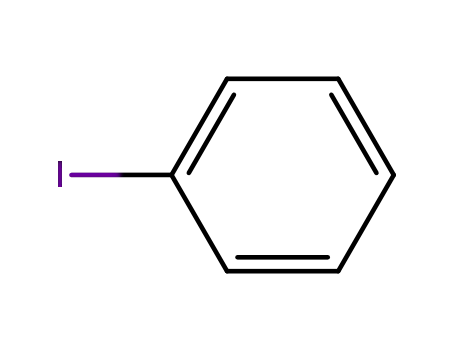
iodobenzene

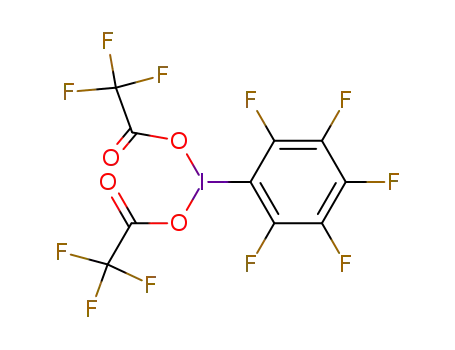
iodopentafluorobenzene bis(trifluoroacetate)

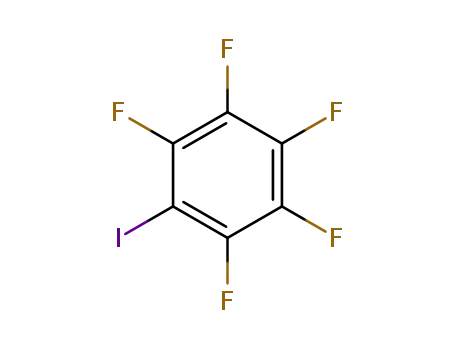
1,2,3,4,5-pentafluoro-6-iodobenzene

![bis-[(trifluoroacetoxy)iodo]benzene](/upload/2025/4/206e18b4-dae3-4d4d-9c84-3f087e5efb89.png)
bis-[(trifluoroacetoxy)iodo]benzene
| Conditions | Yield |
|---|---|
|
With dimethylsulfoxide-d6; at 20 ℃; for 1.58333h; Inert atmosphere;
|
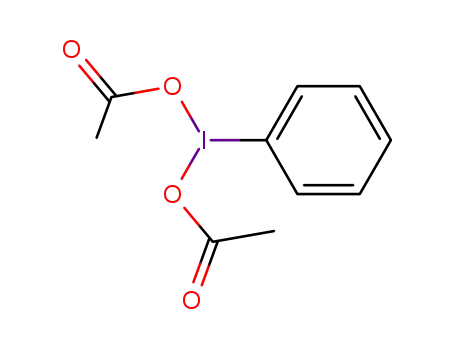
[bis(acetoxy)iodo]benzene
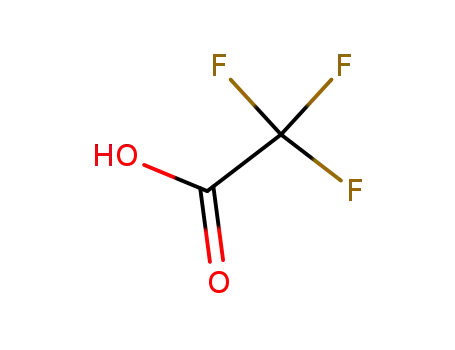
trifluoroacetic acid
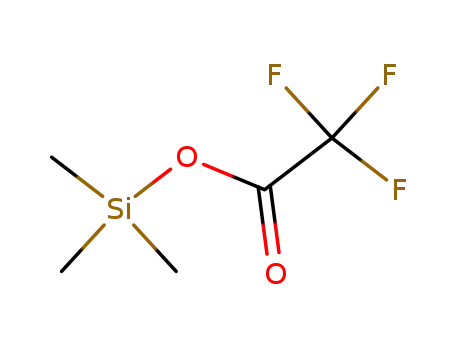
trimethylsilyl trifluoroacetate
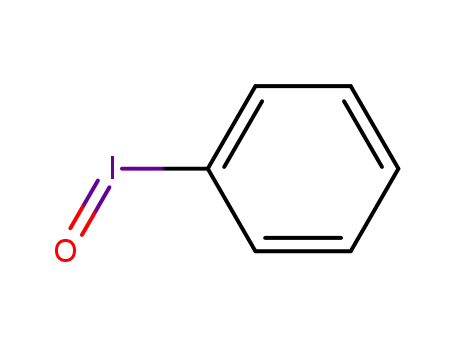
iodosylbenzene
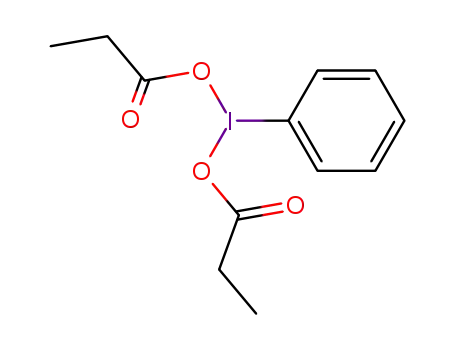
phenyl-λ3-iodanediyl dipropionate
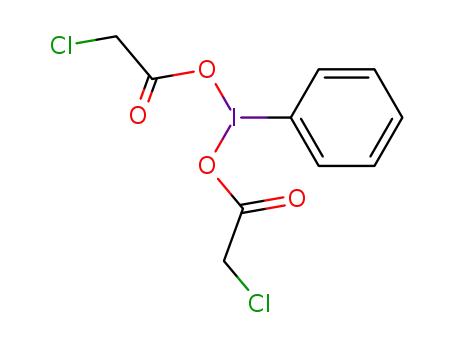
di(chloroacetoxy)iodosobenzene
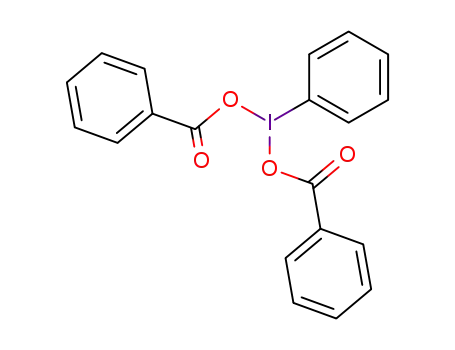
(dibenzoyloxyiodo)benzene
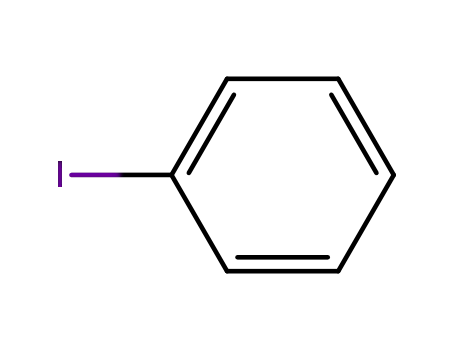
iodobenzene
